当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multicomponent Microscale Biosynthesis of Unnatural Cyanobacterial Indole Alkaloids.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-04-17 , DOI: 10.1021/acssynbio.0c00038 Yogan Khatri , Robert M Hohlman , Johnny Mendoza , Shasha Li , Andrew N Lowell , Haruichi Asahara 1 , David H Sherman
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-04-17 , DOI: 10.1021/acssynbio.0c00038 Yogan Khatri , Robert M Hohlman , Johnny Mendoza , Shasha Li , Andrew N Lowell , Haruichi Asahara 1 , David H Sherman
Affiliation
|
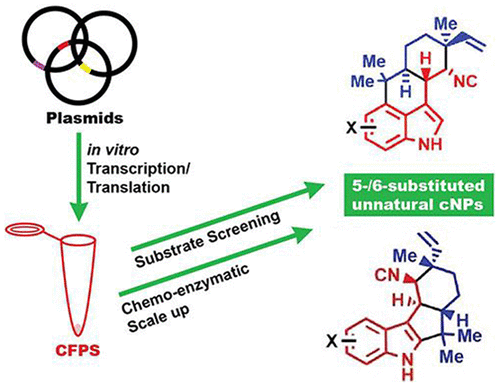
Genome sequencing and bioinformatics tools have facilitated the identification and expression of an increasing number of cryptic biosynthetic gene clusters (BGCs). However, functional analysis of all components of a metabolic pathway to precisely determine biocatalytic properties remains time-consuming and labor intensive. One way to speed this process involves microscale cell-free protein synthesis (CFPS) for direct gene to biochemical function analysis, which has rarely been applied to study multicomponent enzymatic systems in specialized metabolism. We sought to establish an in vitro transcription/translation (TT)-assay to assess assembly of cyanobacterial-derived hapalindole-type natural products (cNPs) because of their diverse bioactivity profiles and complex structural diversity. Using a CFPS system including a plasmid bearing famD2 prenyltransferase from Fischerella ambigua UTEX 1903, we showed production of the central prenylated intermediate (3GC) in the presence of exogenous geranyl-pyrophosphate (GPP) and cis-indole isonitrile. Further addition of a plasmid bearing the famC1 Stig cyclase resulted in synthesis of both FamD2 and FamC1 enzymes, which was confirmed by proteomics analysis, and catalyzed assembly of 12-epi-hapalindole U. Further combinations of Stig cyclases (FamC1–C4) produced hapalindole U and hapalindole H, while FisC identified from Fischerella sp. SAG46.79 generated 12-epi-fischerindole U. The CFPS system was further employed to screen six unnatural halogenated cis-indole isonitrile substrates using FamC1 and FisC, and the reactions were scaled-up using chemoenzymatic synthesis and identified as 5- and 6-fluoro-12-epi-hapalindole U, and 5- and 6-fluoro-12-epi-fischerindole U, respectively. This approach represents an effective, high throughput strategy to determine the functional role of biosynthetic enzymes from diverse natural product BGCs.
中文翻译:

非天然蓝藻吲哚生物碱的多组分微量生物合成。
基因组测序和生物信息学工具促进了越来越多的神秘生物合成基因簇(BGC)的识别和表达。然而,对代谢途径的所有组成部分进行功能分析以精确确定生物催化特性仍然耗时且费力。加速这一过程的一种方法涉及微尺度无细胞蛋白质合成(CFPS),用于直接基因生化功能分析,但很少应用于研究专门代谢中的多组分酶系统。我们试图建立一种体外转录/翻译 (TT) 测定法来评估蓝藻衍生的 hapalindole 型天然产物 (cNP) 的组装,因为它们具有不同的生物活性特征和复杂的结构多样性。使用包含来自Fischerella ambigua UTEX 1903 的带有famD2 异戊烯基转移酶的质粒的 CFPS 系统,我们展示了在外源香叶基焦磷酸 (GPP) 和顺式吲哚异腈存在下中央异戊二烯化中间体 (3GC) 的产生。进一步添加带有famC1 Stig 环化酶的质粒导致 FamD2 和 FamC1 酶的合成,这通过蛋白质组学分析得到证实,并催化 12- epi -hapalindole U 的组装。Stig 环化酶 (FamC1–C4) 的进一步组合产生了 hapalindole U 和 hapalindole H,而 FisC 则从Fischerella sp. 中鉴定出来。 SAG46.79 生成 12-表-费舍吲哚 U。 CFPS 系统进一步使用 FamC1 和 FisC 筛选六种非天然卤代顺式吲哚异腈底物,并使用化学酶合成放大反应,并鉴定为 5- 和 6-氟-12-表-hapalindole U 和 5 -和6-氟-12-表-费舍吲哚U,分别。该方法代表了一种有效的高通量策略,用于确定来自不同天然产物 BGC 的生物合成酶的功能作用。
更新日期:2020-06-19
中文翻译:

非天然蓝藻吲哚生物碱的多组分微量生物合成。
基因组测序和生物信息学工具促进了越来越多的神秘生物合成基因簇(BGC)的识别和表达。然而,对代谢途径的所有组成部分进行功能分析以精确确定生物催化特性仍然耗时且费力。加速这一过程的一种方法涉及微尺度无细胞蛋白质合成(CFPS),用于直接基因生化功能分析,但很少应用于研究专门代谢中的多组分酶系统。我们试图建立一种体外转录/翻译 (TT) 测定法来评估蓝藻衍生的 hapalindole 型天然产物 (cNP) 的组装,因为它们具有不同的生物活性特征和复杂的结构多样性。使用包含来自Fischerella ambigua UTEX 1903 的带有famD2 异戊烯基转移酶的质粒的 CFPS 系统,我们展示了在外源香叶基焦磷酸 (GPP) 和顺式吲哚异腈存在下中央异戊二烯化中间体 (3GC) 的产生。进一步添加带有famC1 Stig 环化酶的质粒导致 FamD2 和 FamC1 酶的合成,这通过蛋白质组学分析得到证实,并催化 12- epi -hapalindole U 的组装。Stig 环化酶 (FamC1–C4) 的进一步组合产生了 hapalindole U 和 hapalindole H,而 FisC 则从Fischerella sp. 中鉴定出来。 SAG46.79 生成 12-表-费舍吲哚 U。 CFPS 系统进一步使用 FamC1 和 FisC 筛选六种非天然卤代顺式吲哚异腈底物,并使用化学酶合成放大反应,并鉴定为 5- 和 6-氟-12-表-hapalindole U 和 5 -和6-氟-12-表-费舍吲哚U,分别。该方法代表了一种有效的高通量策略,用于确定来自不同天然产物 BGC 的生物合成酶的功能作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号