当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyanopyrrolidine Inhibitors of Ubiquitin Specific Protease 7 Mediate Desulfhydration of the Active-Site Cysteine.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-04-17 , DOI: 10.1021/acschembio.0c00031 Charlene Bashore , Priyadarshini Jaishankar 1 , Nicholas J Skelton , Jakob Fuhrmann , Brian R Hearn 1 , Peter S Liu , Adam R Renslo 1 , Erin C Dueber
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-04-17 , DOI: 10.1021/acschembio.0c00031 Charlene Bashore , Priyadarshini Jaishankar 1 , Nicholas J Skelton , Jakob Fuhrmann , Brian R Hearn 1 , Peter S Liu , Adam R Renslo 1 , Erin C Dueber
Affiliation
|
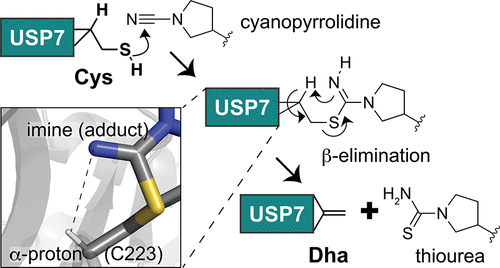
Ubiquitin specific protease 7 (USP7) regulates the protein stability of key cellular regulators in pathways ranging from apoptosis to neuronal development, making it a promising therapeutic target. Here we used an engineered, activated variant of the USP7 catalytic domain to perform structure–activity studies of electrophilic peptidomimetic inhibitors. Employing this USP7 variant, we found that inhibitors with a cyanopyrrolidine warhead unexpectedly promoted a β-elimination reaction of the initial covalent adducts, thereby converting the active-site cysteine residue to dehydroalanine. We determined that this phenomenon is specific for the USP7 catalytic cysteine and that structural features of the inhibitor and protein microenvironment impact elimination rates. Using comprehensive docking studies, we propose that the characteristic conformational dynamics of USP7 allow access to conformations that promote the ligand-induced elimination. Unlike in conventional reversible-covalent inhibition, the compounds described here irreversibly destroy a catalytic residue while simultaneously converting the inhibitor to a nonelectrophilic byproduct. Accordingly, this unexpected finding expands the scope of covalent inhibitor modalities and offers intriguing insights into enzyme–inhibitor dynamics.
中文翻译:

泛素特异性蛋白酶7的氰吡咯烷抑制剂介导活性位点半胱氨酸的脱硫。
泛素特异性蛋白酶7(USP7)调节细胞凋亡,神经元发育等途径中关键细胞调节剂的蛋白质稳定性,使其成为有希望的治疗靶标。在这里,我们使用USP7催化结构域的工程化激活变体进行亲电拟肽抑制剂的结构活性研究。使用这种USP7变体,我们发现具有氰基吡咯烷战斗部的抑制剂意外地促进了初始共价加合物的β-消除反应,从而将活性位点的半胱氨酸残基转化为脱氢丙氨酸。我们确定该现象是USP7催化半胱氨酸特有的,并且抑制剂的结构特征和蛋白质微环境影响消除率。使用全面的对接研究,我们建议USP7的特征构象动力学允许访问促进配体诱导消除的构象。与常规可逆共价抑制不同,此处描述的化合物不可逆地破坏催化残基,同时将抑制剂转化为非亲电子副产物。因此,这一出乎意料的发现扩大了共价抑制剂形式的范围,并为酶抑制剂动力学提供了有趣的见解。
更新日期:2020-06-19
中文翻译:

泛素特异性蛋白酶7的氰吡咯烷抑制剂介导活性位点半胱氨酸的脱硫。
泛素特异性蛋白酶7(USP7)调节细胞凋亡,神经元发育等途径中关键细胞调节剂的蛋白质稳定性,使其成为有希望的治疗靶标。在这里,我们使用USP7催化结构域的工程化激活变体进行亲电拟肽抑制剂的结构活性研究。使用这种USP7变体,我们发现具有氰基吡咯烷战斗部的抑制剂意外地促进了初始共价加合物的β-消除反应,从而将活性位点的半胱氨酸残基转化为脱氢丙氨酸。我们确定该现象是USP7催化半胱氨酸特有的,并且抑制剂的结构特征和蛋白质微环境影响消除率。使用全面的对接研究,我们建议USP7的特征构象动力学允许访问促进配体诱导消除的构象。与常规可逆共价抑制不同,此处描述的化合物不可逆地破坏催化残基,同时将抑制剂转化为非亲电子副产物。因此,这一出乎意料的发现扩大了共价抑制剂形式的范围,并为酶抑制剂动力学提供了有趣的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号