当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling the Residence Time Distribution of Integrated Continuous Bioprocesses.
Biotechnology Journal ( IF 3.2 ) Pub Date : 2020-04-17 , DOI: 10.1002/biot.202000008 Jure Sencar 1 , Nikolaus Hammerschmidt 1 , Alois Jungbauer 1, 2
Biotechnology Journal ( IF 3.2 ) Pub Date : 2020-04-17 , DOI: 10.1002/biot.202000008 Jure Sencar 1 , Nikolaus Hammerschmidt 1 , Alois Jungbauer 1, 2
Affiliation
|
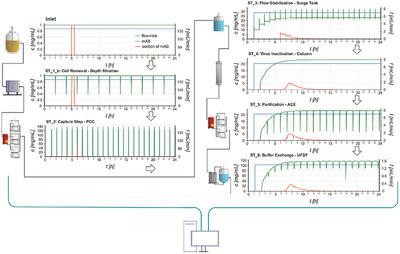
In response to the biopharmaceutical industry advancing from traditional batch operation to continuous operation, the Food and Drug Administration (FDA) has published a draft for continuous integrated biomanufacturing. This draft outlines the most important rules for establishing continuous integration. One of these rules is a thorough understanding of mass flows in the process. A computer simulation framework is developed for modeling the residence time distribution (RTD) of integrated continuous downstream processes based on a unit‐by‐unit modeling approach in which unit operations are simulated one‐by‐one across the entire processing time, and then combined into an integrated RTD model. The framework allows for easy addition or replacement of new unit operations, as well as quick adjustment of process parameters during evaluation of the RTD model. With this RTD model, the start‐up phase to reach steady state can be accelerated, the effects of process disturbances at any stage of the process can be calculated, and virtual tracking of a section of the inlet material throughout the process is possible. A hypothetical biomanufacturing process for an antibody was chosen for showcasing the RTD modeling approach.
中文翻译:

模拟集成连续生物过程的停留时间分布。
为了应对生物制药行业从传统的分批操作向连续操作的发展,美国食品药品监督管理局(FDA)已发布了有关连续集成生物制造的草案。该草案概述了建立持续集成的最重要规则。这些规则之一是对过程中质量流的透彻了解。开发了一种计算机仿真框架,用于基于逐个单元建模方法对集成的连续下游过程的停留时间分布(RTD)进行建模,其中在整个处理时间内逐个模拟单元操作,然后进行组合集成到集成的RTD模型中。该框架可轻松添加或替换新的单元操作,以及在RTD模型评估期间快速调整过程参数。使用此RTD模型,可以加速达到稳态的启动阶段,可以计算出过程中任何阶段的过程干扰的影响,并且可以在整个过程中虚拟跟踪一部分进口物料。选择了一种假设的抗体生物制造过程来展示RTD建模方法。
更新日期:2020-04-17
中文翻译:

模拟集成连续生物过程的停留时间分布。
为了应对生物制药行业从传统的分批操作向连续操作的发展,美国食品药品监督管理局(FDA)已发布了有关连续集成生物制造的草案。该草案概述了建立持续集成的最重要规则。这些规则之一是对过程中质量流的透彻了解。开发了一种计算机仿真框架,用于基于逐个单元建模方法对集成的连续下游过程的停留时间分布(RTD)进行建模,其中在整个处理时间内逐个模拟单元操作,然后进行组合集成到集成的RTD模型中。该框架可轻松添加或替换新的单元操作,以及在RTD模型评估期间快速调整过程参数。使用此RTD模型,可以加速达到稳态的启动阶段,可以计算出过程中任何阶段的过程干扰的影响,并且可以在整个过程中虚拟跟踪一部分进口物料。选择了一种假设的抗体生物制造过程来展示RTD建模方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号