当前位置:
X-MOL 学术
›
Mol. Inform.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Fragment Library of Natural Products and its Comparative Chemoinformatic Characterization.
Molecular Informatics ( IF 2.8 ) Pub Date : 2020-04-17 , DOI: 10.1002/minf.202000050 Ana L Chávez-Hernández 1 , Norberto Sánchez-Cruz 1 , José L Medina-Franco 1
Molecular Informatics ( IF 2.8 ) Pub Date : 2020-04-17 , DOI: 10.1002/minf.202000050 Ana L Chávez-Hernández 1 , Norberto Sánchez-Cruz 1 , José L Medina-Franco 1
Affiliation
|
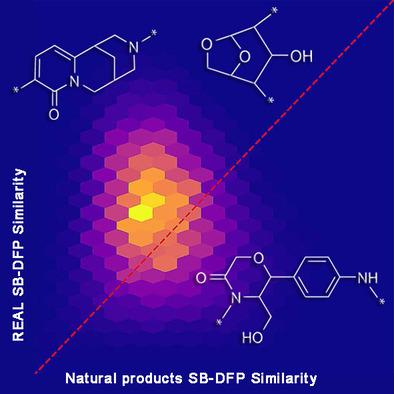
We report a comprehensive fragment library with 205,903 fragments derived from the recently published Collection of Open Natural Products (COCONUT) data set with more than 400,000 non‐redundant natural products. The natural products‐based fragment library was compared with other two fragment libraries herein generated from ChEMBL (biologically relevant compounds) and Enamine‐REAL (a large on‐demand collection of synthetic compounds), both used as reference data sets with relevance in drug discovery. It was found that there is a large diversity of unique fragments derived from natural products and that the entire structures and fragments derived from natural products are more diverse and structurally complex than the two reference compound collections. During this work we introduced a novel visual representation of the chemical space based on the recently published concept of statistical‐based database fingerprint. The compounds and fragments libraries from natural products generated and analyzed in this work are freely available.
中文翻译:

天然产物片段库及其比较化学信息学特征。
我们报告了一个综合片段库,其中包含 205,903 个片段,这些片段来自最近发布的开放天然产物集合 (COCONUT) 数据集,其中包含超过 400,000 个非冗余天然产物。将基于天然产物的片段库与本文中从 ChEMBL(生物相关化合物)和 Enamine-REAL(大量按需合成化合物集合)生成的其他两个片段库进行比较,两者均用作与药物发现相关的参考数据集. 发现来自天然产物的独特片段有很大的多样性,并且来自天然产物的整个结构和片段比两个参考化合物集合更加多样化和结构复杂。在这项工作中,我们基于最近发布的基于统计的数据库指纹概念引入了一种新颖的化学空间视觉表示。在这项工作中生成和分析的天然产物的化合物和片段库是免费提供的。
更新日期:2020-04-17
中文翻译:

天然产物片段库及其比较化学信息学特征。
我们报告了一个综合片段库,其中包含 205,903 个片段,这些片段来自最近发布的开放天然产物集合 (COCONUT) 数据集,其中包含超过 400,000 个非冗余天然产物。将基于天然产物的片段库与本文中从 ChEMBL(生物相关化合物)和 Enamine-REAL(大量按需合成化合物集合)生成的其他两个片段库进行比较,两者均用作与药物发现相关的参考数据集. 发现来自天然产物的独特片段有很大的多样性,并且来自天然产物的整个结构和片段比两个参考化合物集合更加多样化和结构复杂。在这项工作中,我们基于最近发布的基于统计的数据库指纹概念引入了一种新颖的化学空间视觉表示。在这项工作中生成和分析的天然产物的化合物和片段库是免费提供的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号