当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncolytic adenovirus ORCA-010 increases the type 1 T cell stimulatory capacity of melanoma-conditioned dendritic cells.
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-04-17 , DOI: 10.1111/cei.13442 M López González 1 , R van de Ven 1, 2 , H de Haan 1 , J van Eck van der Sluijs 1 , W Dong 3 , V W van Beusechem 1, 3 , T D de Gruijl 1
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-04-17 , DOI: 10.1111/cei.13442 M López González 1 , R van de Ven 1, 2 , H de Haan 1 , J van Eck van der Sluijs 1 , W Dong 3 , V W van Beusechem 1, 3 , T D de Gruijl 1
Affiliation
|
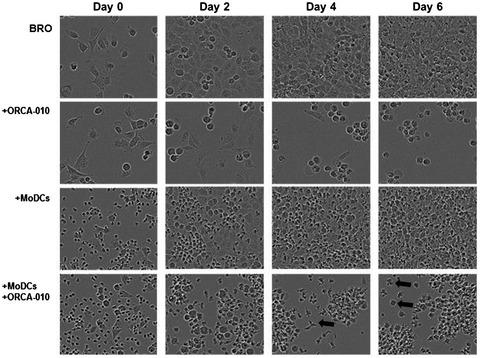
Immune checkpoint blockade has resulted in durable responses in patients with metastatic melanoma, but only in a fraction of treated patients. For immune checkpoint inhibitors (ICI) to be effective, sufficient infiltration with tumor‐reactive T cells is essential. Oncolytic viruses (OV) selectively replicate in and lyse tumor cells and so induce an immunogenic form of cell death, providing at once a source of tumor‐associated (neo)antigens and of danger signals that together induce effective T cell immunity and tumor infiltration. Melanoma‐associated suppression of dendritic cell (DC) differentiation effectively hampers OV‐ or immune checkpoint inhibitor (ICI)‐induced anti‐tumor immunity, due to a consequent inability to prime and attract anti‐tumor effector T cells. Here, we set out to study the effect of ORCA‐010, a clinical stage oncolytic adenovirus, on DC differentiation and functionality in the context of human melanoma. In melanoma and monocyte co‐cultures, employing a panel of five melanoma cell lines with varying origins and oncogenic mutation status, we observed clear suppression of DC development with apparent skewing of monocyte differentiation to a more M2‐macrophage‐like state. We established the ability of ORCA‐010 to productively infect and lyse the melanoma cells. Moreover, although ORCA‐010 was unable to restore DC differentiation, it induced activation and an increased co‐stimulatory capacity of monocyte‐derived antigen‐presenting cells. Their subsequent ability to prime effector T cells with a type I cytokine profile was significantly increased in an allogeneic mixed leukocyte reaction. Our findings suggest that ORCA‐010 is a valuable immunotherapeutic agent for melanoma.
中文翻译:

溶瘤腺病毒ORCA-010增加了黑色素瘤条件树突状细胞的1型T细胞刺激能力。
免疫检查点封锁已导致转移性黑色素瘤患者产生持久反应,但仅在一部分接受治疗的患者中出现。为了使免疫检查点抑制剂(ICI)发挥作用,必须具有足够的肿瘤反应性T细胞浸润。溶瘤病毒(OV)在肿瘤细胞中选择性复制并溶解,从而诱导细胞死亡的免疫原性形式,立即提供肿瘤相关(新)抗原和危险信号的来源,共同诱导有效的T细胞免疫和肿瘤浸润。黑色素瘤相关的树突状细胞(DC)分化抑制有效地阻碍了OV或免疫检查点抑制剂(ICI)诱导的抗肿瘤免疫力,原因是无法启动和吸引抗肿瘤效应T细胞。在这里,我们着手研究ORCA‐010的效果,临床阶段溶瘤腺病毒,涉及人类黑色素瘤背景下的DC分化和功能。在黑色素瘤和单核细胞共培养中,我们使用了五种不同来源和致癌突变状态的黑色素瘤细胞系,观察到DC的明显抑制,单核细胞分化明显偏向了M2巨噬细胞样状态。我们建立了ORCA-010生产性感染和裂解黑色素瘤细胞的能力。而且,尽管ORCA-010无法恢复DC分化,但它诱导了单核细胞衍生的抗原呈递细胞的激活和共刺激能力的增强。在同种异体混合白细胞反应中,它们随后启动具有I型细胞因子特征的效应T细胞的能力显着提高。
更新日期:2020-04-17
中文翻译:

溶瘤腺病毒ORCA-010增加了黑色素瘤条件树突状细胞的1型T细胞刺激能力。
免疫检查点封锁已导致转移性黑色素瘤患者产生持久反应,但仅在一部分接受治疗的患者中出现。为了使免疫检查点抑制剂(ICI)发挥作用,必须具有足够的肿瘤反应性T细胞浸润。溶瘤病毒(OV)在肿瘤细胞中选择性复制并溶解,从而诱导细胞死亡的免疫原性形式,立即提供肿瘤相关(新)抗原和危险信号的来源,共同诱导有效的T细胞免疫和肿瘤浸润。黑色素瘤相关的树突状细胞(DC)分化抑制有效地阻碍了OV或免疫检查点抑制剂(ICI)诱导的抗肿瘤免疫力,原因是无法启动和吸引抗肿瘤效应T细胞。在这里,我们着手研究ORCA‐010的效果,临床阶段溶瘤腺病毒,涉及人类黑色素瘤背景下的DC分化和功能。在黑色素瘤和单核细胞共培养中,我们使用了五种不同来源和致癌突变状态的黑色素瘤细胞系,观察到DC的明显抑制,单核细胞分化明显偏向了M2巨噬细胞样状态。我们建立了ORCA-010生产性感染和裂解黑色素瘤细胞的能力。而且,尽管ORCA-010无法恢复DC分化,但它诱导了单核细胞衍生的抗原呈递细胞的激活和共刺激能力的增强。在同种异体混合白细胞反应中,它们随后启动具有I型细胞因子特征的效应T细胞的能力显着提高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号