当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics and Kinetics of Curing Reaction Between Azide Binder ATP and Bispropargyl Succinate
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-04-17 , DOI: 10.1002/prep.201900302 Na Li 1 , Fengqi Zhao 1 , Ting An 1 , Yanjing Yang 1 , Hui Li 1 , Jiankan Zhang 1 , Ming Zhang 1
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-04-17 , DOI: 10.1002/prep.201900302 Na Li 1 , Fengqi Zhao 1 , Ting An 1 , Yanjing Yang 1 , Hui Li 1 , Jiankan Zhang 1 , Ming Zhang 1
Affiliation
|
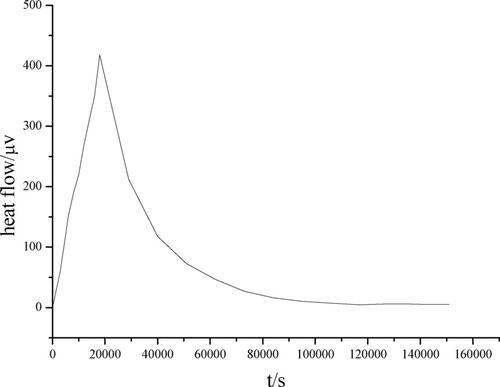
The thermodynamics and kinetics of the curing reactions between azide binder ATP and bispropargyl succinate (BPS) were studied by using a microcalorimeter. On the basis of experimental and calculated results, three thermodynamic parameters (i. e., the activation enthalpies, the activation entropies, the activation free energies), the rate constant, three kinetic parameters (i. e., the activation energies, the pre‐exponential constant and the reaction order) and the enthalpies of the curing reactions between ATP and BPS in the temperature range of 323.15–338.15 K were obtained, showing that the main reaction leading to a triazole structure easily took place in the studied temperature. The linear relationship between lnk and the molar ratio of triple bonds to azide groups was found. Furthermore, the corresponding kinetic equation describing the curing reaction between ATP and BPS was dα/dt=10−4.12(1‐α)1.01 at 333.15 K.
中文翻译:

叠氮化物粘合剂ATP与琥珀酸双炔丙基酯固化反应的热力学和动力学
通过使用微量量热计研究了叠氮化物粘合剂ATP与琥珀酸双炔丙基酯(BPS)之间固化反应的热力学和动力学。根据实验和计算结果,三个热力学参数(即活化焓,活化熵,活化自由能),速率常数,三个动力学参数(即活化能,指数前常数和(反应顺序)和在323.15–338.15 K的温度范围内获得了ATP和BPS之间的固化反应焓,表明在所研究的温度下,导致三唑结构的主要反应很容易发生。ln k之间的线性关系并且发现三键与叠氮化物基团的摩尔比。此外,描述ATP和BPS之间的固化反应的动力学方程为333.15 K下dα/ dt = 10 -4.12(1-α)1.01。
更新日期:2020-04-17
中文翻译:

叠氮化物粘合剂ATP与琥珀酸双炔丙基酯固化反应的热力学和动力学
通过使用微量量热计研究了叠氮化物粘合剂ATP与琥珀酸双炔丙基酯(BPS)之间固化反应的热力学和动力学。根据实验和计算结果,三个热力学参数(即活化焓,活化熵,活化自由能),速率常数,三个动力学参数(即活化能,指数前常数和(反应顺序)和在323.15–338.15 K的温度范围内获得了ATP和BPS之间的固化反应焓,表明在所研究的温度下,导致三唑结构的主要反应很容易发生。ln k之间的线性关系并且发现三键与叠氮化物基团的摩尔比。此外,描述ATP和BPS之间的固化反应的动力学方程为333.15 K下dα/ dt = 10 -4.12(1-α)1.01。











































 京公网安备 11010802027423号
京公网安备 11010802027423号