当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Quinone‐Based Catholytes for Aqueous Redox Flow Batteries and Demonstration of Long‐Term Stability with Tetrasubstituted Quinones
Advanced Energy Materials ( IF 26 ) Pub Date : 2020-04-15 , DOI: 10.1002/aenm.202000340 James B. Gerken , Colin W. Anson , Yuliya Preger , Peter G. Symons , J. David Genders , Yang Qiu , Wenzhen Li , Thatcher W. Root , Shannon S. Stahl
Advanced Energy Materials ( IF 26 ) Pub Date : 2020-04-15 , DOI: 10.1002/aenm.202000340 James B. Gerken , Colin W. Anson , Yuliya Preger , Peter G. Symons , J. David Genders , Yang Qiu , Wenzhen Li , Thatcher W. Root , Shannon S. Stahl
|
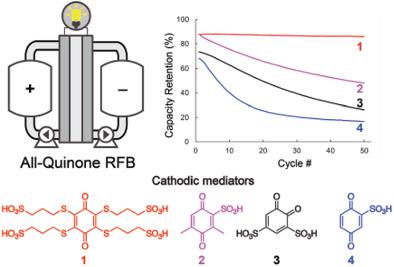
Quinones are appealing targets as organic charge carriers for aqueous redox flow batteries (RFBs), but their utility continues to be constrained by limited stability under operating conditions. The present study evaluates the stability of a series of water‐soluble quinones, with redox potentials ranging from 605–885 mV versus NHE, under acidic aqueous conditions (1 m H2SO4). Four of the quinones are examined as cathodic electrolytes in an aqueous RFB, paired with anthraquinone‐2,7‐disulfonate as the anodic electrolyte. The RFB data complement other solution stability tests and show that the most stable electrolyte is a tetrasubstituted quinone containing four sulfonated thioether substituents. The results highlight the importance of substituting all C–H positions of the quinone in order to maximize the quinone stability and set the stage for design of improved organic electrolytes for aqueous RFBs.
中文翻译:

水性氧化还原液电池用醌基阴极的比较以及四取代醌的长期稳定性证明
醌作为水性氧化还原液流电池(RFB)的有机电荷载体是有吸引力的目标,但在操作条件下有限的稳定性仍然限制了它们的实用性。本研究评估了在酸性水溶液条件下(1 m H 2 SO 4),相对于NHE,氧化还原电位范围为605–885 mV的一系列水溶性醌的稳定性。)。将其中的四个醌作为RFB水溶液中的阴极电解质进行检查,并与蒽醌-2,7-二磺酸盐作为阳极电解质配对。RFB数据补充了其他溶液稳定性测试,表明最稳定的电解质是含有四个磺化硫醚取代基的四取代醌。结果强调了取代醌的所有C–H位置的重要性,以最大程度地提高醌的稳定性,并为改进水性RFB的有机电解质的设计奠定基础。
更新日期:2020-04-15
中文翻译:

水性氧化还原液电池用醌基阴极的比较以及四取代醌的长期稳定性证明
醌作为水性氧化还原液流电池(RFB)的有机电荷载体是有吸引力的目标,但在操作条件下有限的稳定性仍然限制了它们的实用性。本研究评估了在酸性水溶液条件下(1 m H 2 SO 4),相对于NHE,氧化还原电位范围为605–885 mV的一系列水溶性醌的稳定性。)。将其中的四个醌作为RFB水溶液中的阴极电解质进行检查,并与蒽醌-2,7-二磺酸盐作为阳极电解质配对。RFB数据补充了其他溶液稳定性测试,表明最稳定的电解质是含有四个磺化硫醚取代基的四取代醌。结果强调了取代醌的所有C–H位置的重要性,以最大程度地提高醌的稳定性,并为改进水性RFB的有机电解质的设计奠定基础。























































 京公网安备 11010802027423号
京公网安备 11010802027423号