当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cationic Iridium/Chiral Bisphosphine-Catalyzed Enantioselective Hydroacylation of Ketones.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-04-15 , DOI: 10.1002/asia.202000386 Tomohiko Shirai 1 , Tomoya Iwasaki 2 , Kazuya Kanemoto 3 , Yasunori Yamamoto 4
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-04-15 , DOI: 10.1002/asia.202000386 Tomohiko Shirai 1 , Tomoya Iwasaki 2 , Kazuya Kanemoto 3 , Yasunori Yamamoto 4
Affiliation
|
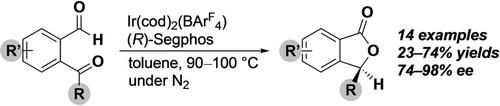
A facile and convenient synthesis of the chiral phthalide framework catalyzed by cationic iridium was developed. The method utilized cationic iridium/bisphosphine‐catalyzed asymmetric intramolecular carbonyl hydroacylation of 2‐keto benzaldehydes to furnish the corresponding optically active phthalide products in good to excellent enantioselectivities (up to 98% ee ). The mechanistic studies using a deuterium‐labelled substrate suggested that the reaction involved an intramolecular carbonyl insertion mechanism to iridium hydride intermediate. In addition, we investigated the kinetic isotope effect (KIE) of intramolecular hydroacylation with deuterated substrate and determined that the C−H activation step is not included in the turnover‐limiting step.
中文翻译:

阳离子铱/手性双膦催化酮的对映选择性加氢酰化反应。
开发了一种方便快捷的阳离子铱催化的手性邻苯二甲酸酯骨架的合成方法。该方法利用阳离子铱/双膦催化的2-酮基苯甲醛的不对称分子内羰基加氢酰化反应,以相应的旋光性苯酞产品提供了良好或优异的对映选择性(最高98%ee)。使用氘标记的底物的机理研究表明,该反应涉及氢化铱中间体的分子内羰基插入机理。此外,我们研究了氘化底物的分子内加氢酰化的动力学同位素效应(KIE),并确定了C-H活化步骤不包括在周转限制步骤中。
更新日期:2020-06-23
中文翻译:

阳离子铱/手性双膦催化酮的对映选择性加氢酰化反应。
开发了一种方便快捷的阳离子铱催化的手性邻苯二甲酸酯骨架的合成方法。该方法利用阳离子铱/双膦催化的2-酮基苯甲醛的不对称分子内羰基加氢酰化反应,以相应的旋光性苯酞产品提供了良好或优异的对映选择性(最高98%ee)。使用氘标记的底物的机理研究表明,该反应涉及氢化铱中间体的分子内羰基插入机理。此外,我们研究了氘化底物的分子内加氢酰化的动力学同位素效应(KIE),并确定了C-H活化步骤不包括在周转限制步骤中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号