当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Key role of macrophages in tolerance induction via T regulatory type 1 (Tr1) cells.
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-04-15 , DOI: 10.1111/cei.13440 B Mfarrej 1 , T Jofra 1 , C Morsiani 1 , N Gagliani 1 , G Fousteri 1 , M Battaglia 1
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-04-15 , DOI: 10.1111/cei.13440 B Mfarrej 1 , T Jofra 1 , C Morsiani 1 , N Gagliani 1 , G Fousteri 1 , M Battaglia 1
Affiliation
|
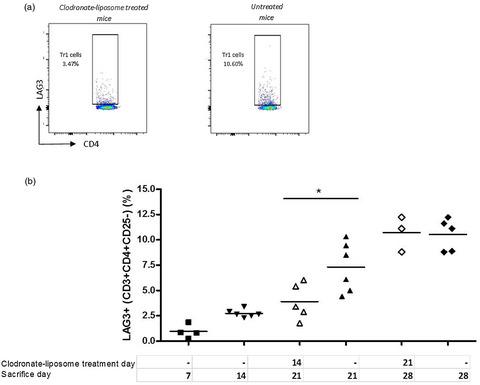
T regulatory type 1 (Tr1) cells are a class of regulatory T cells (Tregs) participating in peripheral tolerance, hence the rationale behind their testing in clinical trials in different disease settings. One of their applications is tolerance induction to allogeneic islets for long‐term diabetes‐free survival. Currently the cellular and molecular mechanisms that promote Tr1‐cell induction in vivo remain poorly understood. We employed a mouse model of transplant tolerance where treatment with granulocyte colony‐stimulating factor (G‐CSF)/rapamycin induces permanent engraftment of allogeneic pancreatic islets in C57BL/6 mice via Tr1 cells. The innate composition of graft and spleen cells in tolerant mice was analyzed by flow cytometry. Graft phagocytic cells were co‐cultured with CD4+ T cells in vitro to test their ability to induce Tr1‐cell induction. Graft phagocytic cells were depleted in vivo at different time‐points during G‐CSF/rapamycin treatment, to identify their role in Tr1‐cell induction and consequently in graft survival. In the spleen, the site of Tr1‐cell induction, no differences in the frequencies of macrophages or dendritic cells (DC) were observed. In the graft, the site of antigen uptake, a high proportion of macrophages and not DC was detected in tolerant but not in rejecting mice. Graft‐infiltrating macrophages of G‐CSF/rapamycin‐treated mice had an M2 phenotype, characterized by higher CD206 expression and interleukin (IL)‐10 production, whereas splenic macrophages only had an increased CD206 expression. Graft‐infiltrating cells from G‐CSF/rapamycin‐treated mice‐induced Tr1‐cell expansion in vitro . Furthermore, Tr1‐cell induction was perturbed upon in‐vivo depletion of phagocytic cells, early and not late during treatment, leading to graft loss suggesting that macrophages play a key role in tolerance induction mediated by Tr1 cells. Taken together, in this mouse model of Tr1‐cell induced tolerance to allogeneic islets, M2 macrophages infiltrating the graft upon G‐CSF/rapamycin treatment are key for Tr1‐cell induction. This work provides mechanistic insight into pharmacologically induced Tr1‐cell expansion in vivo in this stringent model of allogeneic transplantation.
中文翻译:

巨噬细胞在 T 调节 1 型 (Tr1) 细胞耐受诱导中的关键作用。
T 调节型 1 (Tr1) 细胞是一类参与外周耐受的调节性 T 细胞 (T regs ),因此它们在不同疾病环境的临床试验中进行测试的基本原理。它们的应用之一是诱导同种异体胰岛的耐受性,以实现长期无糖尿病生存。目前,在体内促进 Tr1 细胞诱导的细胞和分子机制仍然知之甚少。我们采用了移植耐受性小鼠模型,其中粒细胞集落刺激因子 (G-CSF)/雷帕霉素治疗通过 Tr1 细胞诱导 C57BL/6 小鼠同种异体胰岛的永久性植入。通过流式细胞术分析耐受小鼠中移植物和脾细胞的先天组成。移植吞噬细胞与 CD4 共培养+体外T 细胞以测试其诱导 Tr1 细胞诱导的能力。移植物吞噬细胞在体内耗尽在 G-CSF/雷帕霉素治疗期间的不同时间点,确定它们在 Tr1 细胞诱导和移植物存活中的作用。在 Tr1 细胞诱导部位脾脏中,巨噬细胞或树突细胞 (DC) 的频率没有差异。在移植物中,抗原摄取位点、高比例的巨噬细胞而非 DC 在耐受小鼠中被检测到,但在排斥小鼠中未检测到。G-CSF/雷帕霉素处理的小鼠的移植物浸润巨噬细胞具有 M2 表型,其特征是更高的 CD206 表达和白细胞介素 (IL)-10 产生,而脾脏巨噬细胞仅具有增加的 CD206 表达。来自 G-CSF/雷帕霉素处理的小鼠的移植物浸润细胞诱导的 Tr1 细胞体外扩增。此外,Tr1 细胞诱导在体内受到干扰在治疗期间早期而非晚期吞噬细胞的消耗,导致移植物丢失,表明巨噬细胞在 Tr1 细胞介导的耐受诱导中起关键作用。总之,在这种 Tr1 细胞诱导对同种异体胰岛耐受的小鼠模型中,M2 巨噬细胞在 G-CSF/雷帕霉素处理后浸润移植物是 Tr1 细胞诱导的关键。这项工作提供了在这种严格的同种异体移植模型中体内药理学诱导的 Tr1 细胞扩增的机制见解。
更新日期:2020-04-15
中文翻译:

巨噬细胞在 T 调节 1 型 (Tr1) 细胞耐受诱导中的关键作用。
T 调节型 1 (Tr1) 细胞是一类参与外周耐受的调节性 T 细胞 (T regs ),因此它们在不同疾病环境的临床试验中进行测试的基本原理。它们的应用之一是诱导同种异体胰岛的耐受性,以实现长期无糖尿病生存。目前,在体内促进 Tr1 细胞诱导的细胞和分子机制仍然知之甚少。我们采用了移植耐受性小鼠模型,其中粒细胞集落刺激因子 (G-CSF)/雷帕霉素治疗通过 Tr1 细胞诱导 C57BL/6 小鼠同种异体胰岛的永久性植入。通过流式细胞术分析耐受小鼠中移植物和脾细胞的先天组成。移植吞噬细胞与 CD4 共培养+体外T 细胞以测试其诱导 Tr1 细胞诱导的能力。移植物吞噬细胞在体内耗尽在 G-CSF/雷帕霉素治疗期间的不同时间点,确定它们在 Tr1 细胞诱导和移植物存活中的作用。在 Tr1 细胞诱导部位脾脏中,巨噬细胞或树突细胞 (DC) 的频率没有差异。在移植物中,抗原摄取位点、高比例的巨噬细胞而非 DC 在耐受小鼠中被检测到,但在排斥小鼠中未检测到。G-CSF/雷帕霉素处理的小鼠的移植物浸润巨噬细胞具有 M2 表型,其特征是更高的 CD206 表达和白细胞介素 (IL)-10 产生,而脾脏巨噬细胞仅具有增加的 CD206 表达。来自 G-CSF/雷帕霉素处理的小鼠的移植物浸润细胞诱导的 Tr1 细胞体外扩增。此外,Tr1 细胞诱导在体内受到干扰在治疗期间早期而非晚期吞噬细胞的消耗,导致移植物丢失,表明巨噬细胞在 Tr1 细胞介导的耐受诱导中起关键作用。总之,在这种 Tr1 细胞诱导对同种异体胰岛耐受的小鼠模型中,M2 巨噬细胞在 G-CSF/雷帕霉素处理后浸润移植物是 Tr1 细胞诱导的关键。这项工作提供了在这种严格的同种异体移植模型中体内药理学诱导的 Tr1 细胞扩增的机制见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号