当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate Recognition by the Class II Lanthipeptide Synthetase HalM2.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-04-15 , DOI: 10.1021/acschembio.0c00127 Imran R Rahman 1 , Jeella Z Acedo 2 , Xiaoran Roger Liu 3 , Lingyang Zhu 4 , Justine Arrington 5 , Michael L Gross 3 , Wilfred A van der Donk 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-04-15 , DOI: 10.1021/acschembio.0c00127 Imran R Rahman 1 , Jeella Z Acedo 2 , Xiaoran Roger Liu 3 , Lingyang Zhu 4 , Justine Arrington 5 , Michael L Gross 3 , Wilfred A van der Donk 1, 2
Affiliation
|
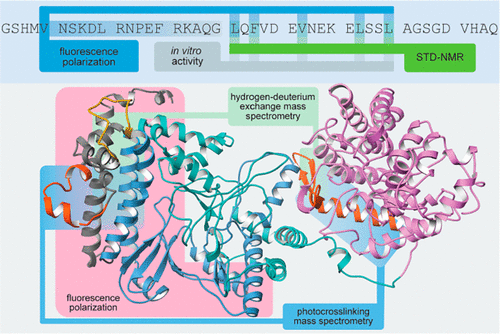
Class II lanthipeptides belong to a diverse group of natural products known as ribosomally synthesized and post-translationally modified peptides (RiPPs). Most RiPP precursor peptides contain an N-terminal recognition sequence known as the leader peptide, which is typically recognized by biosynthetic enzymes that catalyze modifications on the C-terminal core peptide. For class II lanthipeptides, these are carried out by a bifunctional lanthipeptide synthetase (LanM) that catalyzes dehydration and cyclization reactions on peptidic substrates to generate thioether-containing, macrocyclic molecules. Some lanthipeptide synthetases are extraordinarily substrate tolerant, making them promising candidates for biotechnological applications such as combinatorial biosynthesis and cyclic peptide library construction. In this study, we characterized the mode of leader peptide recognition by HalM2, the lanthipeptide synthetase responsible for the production of the antimicrobial peptide haloduracin β. Using NMR spectroscopic techniques, in vitro binding assays, and enzyme activity assays, we identified substrate residues that are important for binding to HalM2 and for post-translational modification of the peptide substrates. Additionally, we provide evidence of the binding site on the enzyme using binding assays with truncated enzyme variants, hydrogen–deuterium exchange mass spectrometry, and photoaffinity labeling. Understanding the mechanism by which lanthipeptide synthetases recognize their substrate will facilitate their use in biotechnology, as well as further our general understanding of how RiPP enzymes recognize their substrates.
中文翻译:

II 类羊毛肽合成酶 HalM2 的底物识别。
II 类羊毛脂肽属于多种天然产物,称为核糖体合成和翻译后修饰肽 (RiPP)。大多数 RiPP 前体肽含有称为前导肽的 N 端识别序列,通常由催化 C 端核心肽修饰的生物合成酶识别。对于 II 类羊毛肽,这些是通过双功能羊毛肽合成酶 (LanM) 进行的,该酶催化肽底物的脱水和环化反应,生成含硫醚的大环分子。一些羊毛脂肽合成酶具有出色的底物耐受性,使它们成为生物技术应用(如组合生物合成和环肽文库构建)的有希望的候选者。在这项研究中,我们表征了 HalM2 识别前导肽的模式,HalM2 是负责生产抗菌肽卤尿苷 β 的羊毛肽合成酶。使用核磁共振光谱技术,在体外结合测定和酶活性测定中,我们确定了对结合 HalM2 和肽底物的翻译后修饰很重要的底物残基。此外,我们使用截断酶变体的结合测定、氢-氘交换质谱法和光亲和标记提供了酶上结合位点的证据。了解羊毛脂肽合成酶识别其底物的机制将有助于它们在生物技术中的应用,以及进一步了解 RiPP 酶如何识别其底物。
更新日期:2020-06-19
中文翻译:

II 类羊毛肽合成酶 HalM2 的底物识别。
II 类羊毛脂肽属于多种天然产物,称为核糖体合成和翻译后修饰肽 (RiPP)。大多数 RiPP 前体肽含有称为前导肽的 N 端识别序列,通常由催化 C 端核心肽修饰的生物合成酶识别。对于 II 类羊毛肽,这些是通过双功能羊毛肽合成酶 (LanM) 进行的,该酶催化肽底物的脱水和环化反应,生成含硫醚的大环分子。一些羊毛脂肽合成酶具有出色的底物耐受性,使它们成为生物技术应用(如组合生物合成和环肽文库构建)的有希望的候选者。在这项研究中,我们表征了 HalM2 识别前导肽的模式,HalM2 是负责生产抗菌肽卤尿苷 β 的羊毛肽合成酶。使用核磁共振光谱技术,在体外结合测定和酶活性测定中,我们确定了对结合 HalM2 和肽底物的翻译后修饰很重要的底物残基。此外,我们使用截断酶变体的结合测定、氢-氘交换质谱法和光亲和标记提供了酶上结合位点的证据。了解羊毛脂肽合成酶识别其底物的机制将有助于它们在生物技术中的应用,以及进一步了解 RiPP 酶如何识别其底物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号