Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Preemptive Therapy vs Antiviral Prophylaxis on Cytomegalovirus Disease in Seronegative Liver Transplant Recipients With Seropositive Donors
JAMA ( IF 63.1 ) Pub Date : 2020-04-14 , DOI: 10.1001/jama.2020.3138 Nina Singh 1, 2 , Drew J Winston 3 , Raymund R Razonable 4 , G Marshall Lyon 5 , Fernanda P Silveira 1 , Marilyn M Wagener 1 , Terry Stevens-Ayers 6 , Bradley Edmison 6 , Michael Boeckh 6 , Ajit P Limaye 7
JAMA ( IF 63.1 ) Pub Date : 2020-04-14 , DOI: 10.1001/jama.2020.3138 Nina Singh 1, 2 , Drew J Winston 3 , Raymund R Razonable 4 , G Marshall Lyon 5 , Fernanda P Silveira 1 , Marilyn M Wagener 1 , Terry Stevens-Ayers 6 , Bradley Edmison 6 , Michael Boeckh 6 , Ajit P Limaye 7
Affiliation
|
Importance
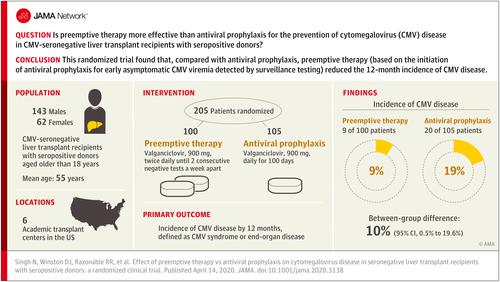
Despite the use of a cytomegalovirus (CMV) prevention strategy of antiviral prophylaxis for high-risk CMV-seronegative liver transplant recipients with seropositive donors, high rates of delayed-onset postprophylaxis CMV disease occur. An alternate approach, preemptive therapy (initiation of antiviral therapy for early asymptomatic CMV viremia detected by surveillance testing), has not previously been directly compared with antiviral prophylaxis in these patients. Objective
To compare preemptive therapy with antiviral prophylaxis in CMV-seronegative liver transplant recipients with seropositive donors for the prevention of CMV disease. Design, Setting, and Participants
Randomized clinical trial of preemptive therapy vs antiviral prophylaxis in 205 CMV-seronegative liver transplant recipients with seropositive donors aged older than 18 years. The trial was conducted at 6 academic transplant centers in the United States between October 2012 and June 2017, with last follow-up in June 2018. Interventions
Patients were randomized 1:1 to receive either preemptive therapy (valganciclovir, 900 mg, twice daily until 2 consecutive negative tests a week apart) for viremia detected by weekly plasma CMV polymerase chain reaction for 100 days (n = 100) or valganciclovir, 900 mg, daily for 100 days as antiviral prophylaxis (n = 105). Main Outcomes and Measures
The primary outcome was incidence of CMV disease by 12 months, defined as CMV syndrome (CMV viremia and clinical or laboratory findings) or end-organ disease. Secondary outcomes included acute allograft rejection, opportunistic infections, graft and patient survival, and neutropenia. Results
Among 205 patients who were randomized (mean age, 55 years; 62 women [30%]), all 205 (100%) completed the trial. The incidence of CMV disease was significantly lower with preemptive therapy than antiviral prophylaxis (9% [9/100] vs 19% [20/105]; difference, 10% [95% CI, 0.5% to 19.6%]; P = .04]). The incidence of allograft rejection (28% vs 25%; difference, 3% [95% CI, -9% to 15%]), opportunistic infections (25% vs 27%; difference, 2% [95% CI, -14% to 10%]), graft loss (2% vs 2%; difference, <1% [95% CI, -4% to 4%]), and neutropenia (13% vs 10%; difference, 3% [95% CI, -5% to 12%]) did not differ significantly for the preemptive therapy vs antiviral prophylaxis group, respectively. All-cause mortality at last follow-up was 15% in the preemptive therapy vs 19% in the antiviral prophylaxis group (difference, 4% [95% CI, -14% to 6%]; P = .46). Conclusions and Relevance
Among CMV-seronegative liver transplant recipients with seropositive donors, the use of preemptive therapy, compared with antiviral prophylaxis, resulted in a lower incidence of CMV disease over 12 months. Further research is needed to replicate these findings and assess long-term outcomes. Trial Registration
ClinicalTrials.gov Identifier: NCT01552369.
中文翻译:

超前治疗与抗病毒预防对血清反应阳性供者的血清阴性肝移植受者巨细胞病毒病的影响
重要性 尽管对血清阳性供者的高危 CMV 血清阴性肝移植受者使用了巨细胞病毒 (CMV) 预防策略进行抗病毒预防,但迟发性预防后 CMV 疾病的发生率仍然很高。另一种方法,即先发制人的治疗(对通过监测检测检测到的早期无症状 CMV 病毒血症开始抗病毒治疗),以前没有直接与这些患者的抗病毒预防治疗进行比较。目的 比较 CMV 血清阴性肝移植受者和血清阳性供者的超前治疗与抗病毒预防治疗预防 CMV 疾病的效果。设计,设置,和参与者 在 205 名 CMV 血清反应阴性的肝移植受者和 18 岁以上的血清阳性供者中进行的抢先治疗与抗病毒预防的随机临床试验。该试验于 2012 年 10 月至 2017 年 6 月在美国的 6 个学术移植中心进行,最后一次随访于 2018 年 6 月。通过每周血浆 CMV 聚合酶链反应检测 100 天(n = 100)或缬更昔洛韦,900 毫克,每天 100 天作为抗病毒预防(n = 105)检测到病毒血症,间隔一周连续 2 次阴性。主要结果和措施 主要结果是 12 个月时 CMV 疾病的发生率,定义为 CMV 综合征(CMV 病毒血症和临床或实验室检查结果)或终末器官疾病。次要结果包括急性同种异体移植排斥、机会性感染、移植物和患者存活率以及中性粒细胞减少症。结果 在随机分组的 205 名患者中(平均年龄 55 岁;62 名女性 [30%]),所有 205 名 (100%) 完成了试验。抢先治疗的 CMV 发病率显着低于抗病毒预防(9% [9/100] vs 19% [20/105];差异,10% [95% CI,0.5% 到 19.6%];P = . 04])。同种异体移植排斥的发生率(28% vs 25%;差异,3% [95% CI,-9% 至 15%]),机会性感染(25% vs 27%;差异,2% [95% CI,-14 % 至 10%])、移植物丢失(2% 与 2%;差异,<1% [95% CI,-4% 至 4%])和中性粒细胞减少症(13% 与 10%;差异,3% [95 % CI, -5% 至 12%]) 分别对于抢先治疗与抗病毒预防组没有显着差异。在最后一次随访时,抢先治疗组的全因死亡率为 15%,而抗病毒预防组为 19%(差异,4% [95% CI,-14% 至 6%];P = .46)。结论和相关性 在有血清阳性供者的 CMV 血清阴性肝移植受者中,与抗病毒预防性治疗相比,使用抢先治疗可降低 12 个月内 CMV 疾病的发生率。需要进一步的研究来复制这些发现并评估长期结果。试验注册 ClinicalTrials.gov 标识符:NCT01552369。4% [95% CI,-14% 至 6%];P = .46)。结论和相关性 在有血清阳性供者的 CMV 血清阴性肝移植受者中,与抗病毒预防性治疗相比,使用抢先治疗可降低 12 个月内 CMV 疾病的发生率。需要进一步的研究来复制这些发现并评估长期结果。试验注册 ClinicalTrials.gov 标识符:NCT01552369。4% [95% CI,-14% 至 6%];P = .46)。结论和相关性 在有血清阳性供者的 CMV 血清阴性肝移植受者中,与抗病毒预防性治疗相比,使用抢先治疗可降低 12 个月内 CMV 疾病的发生率。需要进一步的研究来复制这些发现并评估长期结果。试验注册 ClinicalTrials.gov 标识符:NCT01552369。
更新日期:2020-04-14
中文翻译:

超前治疗与抗病毒预防对血清反应阳性供者的血清阴性肝移植受者巨细胞病毒病的影响
重要性 尽管对血清阳性供者的高危 CMV 血清阴性肝移植受者使用了巨细胞病毒 (CMV) 预防策略进行抗病毒预防,但迟发性预防后 CMV 疾病的发生率仍然很高。另一种方法,即先发制人的治疗(对通过监测检测检测到的早期无症状 CMV 病毒血症开始抗病毒治疗),以前没有直接与这些患者的抗病毒预防治疗进行比较。目的 比较 CMV 血清阴性肝移植受者和血清阳性供者的超前治疗与抗病毒预防治疗预防 CMV 疾病的效果。设计,设置,和参与者 在 205 名 CMV 血清反应阴性的肝移植受者和 18 岁以上的血清阳性供者中进行的抢先治疗与抗病毒预防的随机临床试验。该试验于 2012 年 10 月至 2017 年 6 月在美国的 6 个学术移植中心进行,最后一次随访于 2018 年 6 月。通过每周血浆 CMV 聚合酶链反应检测 100 天(n = 100)或缬更昔洛韦,900 毫克,每天 100 天作为抗病毒预防(n = 105)检测到病毒血症,间隔一周连续 2 次阴性。主要结果和措施 主要结果是 12 个月时 CMV 疾病的发生率,定义为 CMV 综合征(CMV 病毒血症和临床或实验室检查结果)或终末器官疾病。次要结果包括急性同种异体移植排斥、机会性感染、移植物和患者存活率以及中性粒细胞减少症。结果 在随机分组的 205 名患者中(平均年龄 55 岁;62 名女性 [30%]),所有 205 名 (100%) 完成了试验。抢先治疗的 CMV 发病率显着低于抗病毒预防(9% [9/100] vs 19% [20/105];差异,10% [95% CI,0.5% 到 19.6%];P = . 04])。同种异体移植排斥的发生率(28% vs 25%;差异,3% [95% CI,-9% 至 15%]),机会性感染(25% vs 27%;差异,2% [95% CI,-14 % 至 10%])、移植物丢失(2% 与 2%;差异,<1% [95% CI,-4% 至 4%])和中性粒细胞减少症(13% 与 10%;差异,3% [95 % CI, -5% 至 12%]) 分别对于抢先治疗与抗病毒预防组没有显着差异。在最后一次随访时,抢先治疗组的全因死亡率为 15%,而抗病毒预防组为 19%(差异,4% [95% CI,-14% 至 6%];P = .46)。结论和相关性 在有血清阳性供者的 CMV 血清阴性肝移植受者中,与抗病毒预防性治疗相比,使用抢先治疗可降低 12 个月内 CMV 疾病的发生率。需要进一步的研究来复制这些发现并评估长期结果。试验注册 ClinicalTrials.gov 标识符:NCT01552369。4% [95% CI,-14% 至 6%];P = .46)。结论和相关性 在有血清阳性供者的 CMV 血清阴性肝移植受者中,与抗病毒预防性治疗相比,使用抢先治疗可降低 12 个月内 CMV 疾病的发生率。需要进一步的研究来复制这些发现并评估长期结果。试验注册 ClinicalTrials.gov 标识符:NCT01552369。4% [95% CI,-14% 至 6%];P = .46)。结论和相关性 在有血清阳性供者的 CMV 血清阴性肝移植受者中,与抗病毒预防性治疗相比,使用抢先治疗可降低 12 个月内 CMV 疾病的发生率。需要进一步的研究来复制这些发现并评估长期结果。试验注册 ClinicalTrials.gov 标识符:NCT01552369。











































 京公网安备 11010802027423号
京公网安备 11010802027423号