Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of a Chikungunya Virus–Like Particle Vaccine on Safety and Tolerability Outcomes
JAMA ( IF 63.1 ) Pub Date : 2020-04-14 , DOI: 10.1001/jama.2020.2477 Grace L Chen 1 , Emily E Coates 1 , Sarah H Plummer 1 , Cristina A Carter 1 , Nina Berkowitz 1 , Michelle Conan-Cibotti 1 , Josephine H Cox 1 , Allison Beck 1 , Mark O'Callahan 1 , Charla Andrews 1 , Ingelise J Gordon 1 , Brenda Larkin 1 , Rebecca Lampley 1 , Florence Kaltovich 1 , Jason Gall 1 , Kevin Carlton 1 , Jason Mendy 2 , Doug Haney 2 , Jeanine May 3 , Amy Bray 3 , Robert T Bailer 1 , Kimberly A Dowd 4 , Brittanie Brockett 4 , David Gordon 4 , Richard A Koup 1 , Richard Schwartz 1 , John R Mascola 1 , Barney S Graham 1 , Theodore C Pierson 4 , Yeycy Donastorg 5 , Nicolas Rosario 6 , Jean William Pape 7 , Bruno Hoen 8 , André Cabié 9 , Clemente Diaz 10, 11 , Julie E Ledgerwood 1 ,
JAMA ( IF 63.1 ) Pub Date : 2020-04-14 , DOI: 10.1001/jama.2020.2477 Grace L Chen 1 , Emily E Coates 1 , Sarah H Plummer 1 , Cristina A Carter 1 , Nina Berkowitz 1 , Michelle Conan-Cibotti 1 , Josephine H Cox 1 , Allison Beck 1 , Mark O'Callahan 1 , Charla Andrews 1 , Ingelise J Gordon 1 , Brenda Larkin 1 , Rebecca Lampley 1 , Florence Kaltovich 1 , Jason Gall 1 , Kevin Carlton 1 , Jason Mendy 2 , Doug Haney 2 , Jeanine May 3 , Amy Bray 3 , Robert T Bailer 1 , Kimberly A Dowd 4 , Brittanie Brockett 4 , David Gordon 4 , Richard A Koup 1 , Richard Schwartz 1 , John R Mascola 1 , Barney S Graham 1 , Theodore C Pierson 4 , Yeycy Donastorg 5 , Nicolas Rosario 6 , Jean William Pape 7 , Bruno Hoen 8 , André Cabié 9 , Clemente Diaz 10, 11 , Julie E Ledgerwood 1 ,
Affiliation
|
Importance
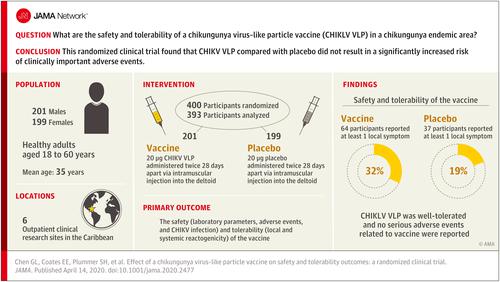
Chikungunya virus (CHIKV) is a mosquito-borne Alphavirus prevalent worldwide. There are currently no licensed vaccines or therapies. Objective
To evaluate the safety and tolerability of an investigational CHIKV virus-like particle (VLP) vaccine in endemic regions. Design, Setting, and Participants
This was a randomized, placebo-controlled, double-blind, phase 2 clinical trial to assess the vaccine VRC-CHKVLP059-00-VP (CHIKV VLP). The trial was conducted at 6 outpatient clinical research sites located in Haiti, Dominican Republic, Martinique, Guadeloupe, and Puerto Rico. A total of 400 healthy adults aged 18 through 60 years were enrolled after meeting eligibility criteria. The first study enrollment occurred on November 18, 2015; the final study visit, March 6, 2018. Interventions
Participants were randomized 1:1 to receive 2 intramuscular injections 28 days apart (20 µg, n = 201) or placebo (n = 199) and were followed up for 72 weeks. Main Outcomes and Measures
The primary outcome was the safety (laboratory parameters, adverse events, and CHIKV infection) and tolerability (local and systemic reactogenicity) of the vaccine, and the secondary outcome was immune response by neutralization assay 4 weeks after second vaccination. Results
Of the 400 randomized participants (mean age, 35 years; 199 [50%] women), 393 (98%) completed the primary safety analysis. All injections were well tolerated. Of the 16 serious adverse events unrelated to the study drugs, 4 (25%) occurred among 4 patients in the vaccine group and 12 (75%) occurred among 11 patients in the placebo group. Of the 16 mild to moderate unsolicited adverse events that were potentially related to the drug, 12 (75%) occurred among 8 patients in the vaccine group and 4 (25%) occurred among 3 patients in the placebo group. All potentially related adverse events resolved without clinical sequelae. At baseline, there was no significant difference between the effective concentration (EC50)-which is the dilution of sera that inhibits 50% infection in viral neutralization assay-geometric mean titers (GMTs) of neutralizing antibodies of the vaccine group (46; 95% CI, 34-63) and the placebo group (43; 95% CI, 32-57). Eight weeks following the first administration, the EC50 GMT in the vaccine group was 2005 (95% CI, 1680-2392) vs 43 (95% CI, 32-58; P < .001) in the placebo group. Durability of the immune response was demonstrated through 72 weeks after vaccination. Conclusions and Relevance
Among healthy adults in a chikungunya endemic population, a virus-like particle vaccine compared with placebo demonstrated safety and tolerability. Phase 3 trials are needed to assess clinical efficacy. Trial Registration
ClinicalTrials.gov Identifier: NCT02562482.
中文翻译:

基孔肯雅病毒样颗粒疫苗对安全性和耐受性结果的影响
重要性 基孔肯雅病毒 (CHIKV) 是一种全球流行的蚊媒甲病毒。目前没有获得许可的疫苗或疗法。目的评价一种在研CHIKV病毒样颗粒(VLP)疫苗在流行地区的安全性和耐受性。设计、设置和参与者 这是一项随机、安慰剂对照、双盲、2 期临床试验,用于评估疫苗 VRC-CHKVLP059-00-VP (CHIKV VLP)。该试验在位于海地、多米尼加共和国、马提尼克岛、瓜德罗普岛和波多黎各的 6 个门诊临床研究中心进行。在符合资格标准后,共有 400 名 18 至 60 岁的健康成年人入组。2015年11月18日首次入组;最后一次研究访问,2018 年 3 月 6 日。 干预 参与者被随机分配 1:1 接受 2 次肌肉注射,间隔 28 天(20 µg,n = 201)或安慰剂(n = 199)并随访 72 周。主要结果和措施主要结果是疫苗的安全性(实验室参数、不良事件和 CHIKV 感染)和耐受性(局部和全身反应原性),次要结果是第二次接种后 4 周通过中和试验得出的免疫反应。结果 在 400 名随机参与者(平均年龄 35 岁;199 名 [50%] 女性)中,393 名 (98%) 完成了主要安全性分析。所有注射均耐受良好。在与研究药物无关的 16 起严重不良事件中,疫苗组的 4 名患者中发生了 4 起(25%),安慰剂组的 11 名患者中发生了 12 起(75%)。在可能与该药物相关的 16 起轻度至中度主动提供的不良事件中,12 起 (75%) 发生在疫苗组的 8 名患者中,4 起 (25%) 发生在安慰剂组的 3 名患者中。所有潜在相关的不良事件都得到解决,没有临床后遗症。在基线时,有效浓度 (EC50)-病毒中和试验中抑制 50% 感染的血清稀释度-疫苗组中和抗体的几何平均滴度 (GMT) (46;95%) 之间没有显着差异CI, 34-63) 和安慰剂组 (43; 95% CI, 32-57)。第一次给药后 8 周,疫苗组的 EC50 GMT 为 2005(95% CI,1680-2392),而安慰剂组为 43(95% CI,32-58;P < .001)。免疫反应的持久性在疫苗接种后 72 周内得到证明。结论和相关性 在基孔肯雅热流行人群中的健康成年人中,与安慰剂相比,病毒样颗粒疫苗显示出安全性和耐受性。需要进行 3 期试验来评估临床疗效。试验注册 ClinicalTrials.gov 标识符:NCT02562482。
更新日期:2020-04-14
中文翻译:

基孔肯雅病毒样颗粒疫苗对安全性和耐受性结果的影响
重要性 基孔肯雅病毒 (CHIKV) 是一种全球流行的蚊媒甲病毒。目前没有获得许可的疫苗或疗法。目的评价一种在研CHIKV病毒样颗粒(VLP)疫苗在流行地区的安全性和耐受性。设计、设置和参与者 这是一项随机、安慰剂对照、双盲、2 期临床试验,用于评估疫苗 VRC-CHKVLP059-00-VP (CHIKV VLP)。该试验在位于海地、多米尼加共和国、马提尼克岛、瓜德罗普岛和波多黎各的 6 个门诊临床研究中心进行。在符合资格标准后,共有 400 名 18 至 60 岁的健康成年人入组。2015年11月18日首次入组;最后一次研究访问,2018 年 3 月 6 日。 干预 参与者被随机分配 1:1 接受 2 次肌肉注射,间隔 28 天(20 µg,n = 201)或安慰剂(n = 199)并随访 72 周。主要结果和措施主要结果是疫苗的安全性(实验室参数、不良事件和 CHIKV 感染)和耐受性(局部和全身反应原性),次要结果是第二次接种后 4 周通过中和试验得出的免疫反应。结果 在 400 名随机参与者(平均年龄 35 岁;199 名 [50%] 女性)中,393 名 (98%) 完成了主要安全性分析。所有注射均耐受良好。在与研究药物无关的 16 起严重不良事件中,疫苗组的 4 名患者中发生了 4 起(25%),安慰剂组的 11 名患者中发生了 12 起(75%)。在可能与该药物相关的 16 起轻度至中度主动提供的不良事件中,12 起 (75%) 发生在疫苗组的 8 名患者中,4 起 (25%) 发生在安慰剂组的 3 名患者中。所有潜在相关的不良事件都得到解决,没有临床后遗症。在基线时,有效浓度 (EC50)-病毒中和试验中抑制 50% 感染的血清稀释度-疫苗组中和抗体的几何平均滴度 (GMT) (46;95%) 之间没有显着差异CI, 34-63) 和安慰剂组 (43; 95% CI, 32-57)。第一次给药后 8 周,疫苗组的 EC50 GMT 为 2005(95% CI,1680-2392),而安慰剂组为 43(95% CI,32-58;P < .001)。免疫反应的持久性在疫苗接种后 72 周内得到证明。结论和相关性 在基孔肯雅热流行人群中的健康成年人中,与安慰剂相比,病毒样颗粒疫苗显示出安全性和耐受性。需要进行 3 期试验来评估临床疗效。试验注册 ClinicalTrials.gov 标识符:NCT02562482。











































 京公网安备 11010802027423号
京公网安备 11010802027423号