Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Unfolded Protein Response Mediator PERK Governs Myeloid Cell-Driven Immunosuppression in Tumors through Inhibition of STING Signaling.
Immunity ( IF 25.5 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.immuni.2020.03.004 Eslam Mohamed 1 , Rosa A Sierra 1 , Jimena Trillo-Tinoco 2 , Yu Cao 1 , Patrick Innamarato 1 , Kyle K Payne 1 , Alvaro de Mingo Pulido 1 , Jessica Mandula 1 , Shuzhong Zhang 3 , Paul Thevenot 4 , Subir Biswas 1 , Sarah K Abdalla 1 , Tara Lee Costich 1 , Kay Hänggi 1 , Carmen M Anadon 1 , Elsa R Flores 5 , Eric B Haura 5 , Shikhar Mehrotra 6 , Shari Pilon-Thomas 1 , Brian Ruffell 1 , David H Munn 7 , Juan R Cubillos-Ruiz 8 , Jose R Conejo-Garcia 1 , Paulo C Rodriguez 1
Immunity ( IF 25.5 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.immuni.2020.03.004 Eslam Mohamed 1 , Rosa A Sierra 1 , Jimena Trillo-Tinoco 2 , Yu Cao 1 , Patrick Innamarato 1 , Kyle K Payne 1 , Alvaro de Mingo Pulido 1 , Jessica Mandula 1 , Shuzhong Zhang 3 , Paul Thevenot 4 , Subir Biswas 1 , Sarah K Abdalla 1 , Tara Lee Costich 1 , Kay Hänggi 1 , Carmen M Anadon 1 , Elsa R Flores 5 , Eric B Haura 5 , Shikhar Mehrotra 6 , Shari Pilon-Thomas 1 , Brian Ruffell 1 , David H Munn 7 , Juan R Cubillos-Ruiz 8 , Jose R Conejo-Garcia 1 , Paulo C Rodriguez 1
Affiliation
|
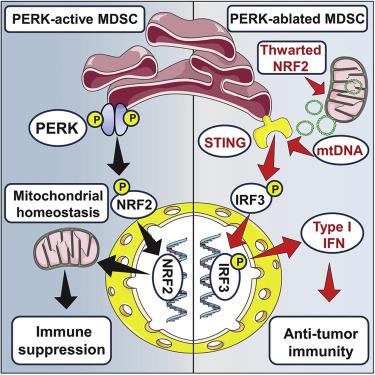
The primary mechanisms supporting immunoregulatory polarization of myeloid cells upon infiltration into tumors remain largely unexplored. Elucidation of these signals could enable better strategies to restore protective anti-tumor immunity. Here, we investigated the role of the intrinsic activation of the PKR-like endoplasmic reticulum (ER) kinase (PERK) in the immunoinhibitory actions of tumor-associated myeloid-derived suppressor cells (tumor-MDSCs). PERK signaling increased in tumor-MDSCs, and its deletion transformed MDSCs into myeloid cells that activated CD8+ T cell-mediated immunity against cancer. Tumor-MDSCs lacking PERK exhibited disrupted NRF2-driven antioxidant capacity and impaired mitochondrial respiratory homeostasis. Moreover, reduced NRF2 signaling in PERK-deficient MDSCs elicited cytosolic mitochondrial DNA elevation and, consequently, STING-dependent expression of anti-tumor type I interferon. Reactivation of NRF2 signaling, conditional deletion of STING, or blockade of type I interferon receptor I restored the immunoinhibitory potential of PERK-ablated MDSCs. Our findings demonstrate the pivotal role of PERK in tumor-MDSC functionality and unveil strategies to reprogram immunosuppressive myelopoiesis in tumors to boost cancer immunotherapy.
中文翻译:

展开的蛋白质反应介体PERK通过抑制STING信号传导来控制肿瘤细胞中的髓样细胞驱动的免疫抑制。
在渗透到肿瘤中时支持髓样细胞的免疫调节极化的主要机制仍未得到充分探索。这些信号的阐明可以使更好的策略来恢复保护性抗肿瘤免疫力。在这里,我们调查了PKR样内质网(ER)激酶(PERK)的内在激活在肿瘤相关的髓样来源的抑制细胞(tumor-MDSCs)的免疫抑制作用中的作用。PERK信号在肿瘤MDSCs中增加,其缺失将MDSCs转变为激活CD8 + T细胞介导的抗癌免疫力的髓样细胞。缺乏PERK的肿瘤MDSCs破坏了NRF2驱动的抗氧化能力并破坏了线粒体呼吸稳态。此外,PERK缺失的MDSC中NRF2信号的减少引起胞浆线粒体DNA升高,因此,STING依赖型抗肿瘤I型干扰素的表达。NRF2信号的重新激活,STING的条件缺失或I型干扰素受体I的阻滞恢复了PERK切除的MDSC的免疫抑制潜能。我们的发现证明了PERK在肿瘤MDSC功能中的关键作用,并揭示了重新编程肿瘤中免疫抑制性骨髓生成以增强癌症免疫疗法的策略。
更新日期:2020-04-21
中文翻译:

展开的蛋白质反应介体PERK通过抑制STING信号传导来控制肿瘤细胞中的髓样细胞驱动的免疫抑制。
在渗透到肿瘤中时支持髓样细胞的免疫调节极化的主要机制仍未得到充分探索。这些信号的阐明可以使更好的策略来恢复保护性抗肿瘤免疫力。在这里,我们调查了PKR样内质网(ER)激酶(PERK)的内在激活在肿瘤相关的髓样来源的抑制细胞(tumor-MDSCs)的免疫抑制作用中的作用。PERK信号在肿瘤MDSCs中增加,其缺失将MDSCs转变为激活CD8 + T细胞介导的抗癌免疫力的髓样细胞。缺乏PERK的肿瘤MDSCs破坏了NRF2驱动的抗氧化能力并破坏了线粒体呼吸稳态。此外,PERK缺失的MDSC中NRF2信号的减少引起胞浆线粒体DNA升高,因此,STING依赖型抗肿瘤I型干扰素的表达。NRF2信号的重新激活,STING的条件缺失或I型干扰素受体I的阻滞恢复了PERK切除的MDSC的免疫抑制潜能。我们的发现证明了PERK在肿瘤MDSC功能中的关键作用,并揭示了重新编程肿瘤中免疫抑制性骨髓生成以增强癌症免疫疗法的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号