Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides.
Immunity ( IF 25.5 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.immuni.2020.03.005 Alexander Perniss 1 , Shuya Liu 2 , Brett Boonen 3 , Maryam Keshavarz 1 , Anna-Lena Ruppert 4 , Thomas Timm 5 , Uwe Pfeil 1 , Aichurek Soultanova 1 , Soumya Kusumakshi 6 , Lucas Delventhal 1 , Öznur Aydin 1 , Martina Pyrski 3 , Klaus Deckmann 1 , Torsten Hain 7 , Nadine Schmidt 8 , Christa Ewers 8 , Andreas Günther 9 , Günter Lochnit 5 , Vladimir Chubanov 10 , Thomas Gudermann 10 , Johannes Oberwinkler 11 , Jochen Klein 12 , Katsuhiko Mikoshiba 13 , Trese Leinders-Zufall 3 , Stefan Offermanns 14 , Burkhard Schütz 4 , Ulrich Boehm 6 , Frank Zufall 3 , Bernd Bufe 15 , Wolfgang Kummer 1
Immunity ( IF 25.5 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.immuni.2020.03.005 Alexander Perniss 1 , Shuya Liu 2 , Brett Boonen 3 , Maryam Keshavarz 1 , Anna-Lena Ruppert 4 , Thomas Timm 5 , Uwe Pfeil 1 , Aichurek Soultanova 1 , Soumya Kusumakshi 6 , Lucas Delventhal 1 , Öznur Aydin 1 , Martina Pyrski 3 , Klaus Deckmann 1 , Torsten Hain 7 , Nadine Schmidt 8 , Christa Ewers 8 , Andreas Günther 9 , Günter Lochnit 5 , Vladimir Chubanov 10 , Thomas Gudermann 10 , Johannes Oberwinkler 11 , Jochen Klein 12 , Katsuhiko Mikoshiba 13 , Trese Leinders-Zufall 3 , Stefan Offermanns 14 , Burkhard Schütz 4 , Ulrich Boehm 6 , Frank Zufall 3 , Bernd Bufe 15 , Wolfgang Kummer 1
Affiliation
|
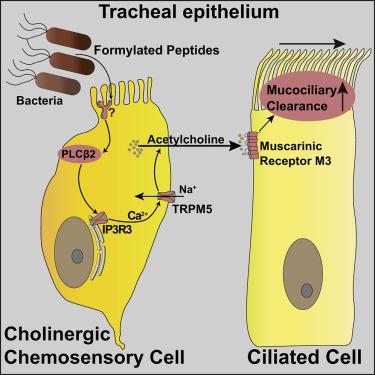
Mucociliary clearance through coordinated ciliary beating is a major innate defense removing pathogens from the lower airways, but the pathogen sensing and downstream signaling mechanisms remain unclear. We identified virulence-associated formylated bacterial peptides that potently stimulated ciliary-driven transport in the mouse trachea. This innate response was independent of formyl peptide and taste receptors but depended on key taste transduction genes. Tracheal cholinergic chemosensory cells expressed these genes, and genetic ablation of these cells abrogated peptide-driven stimulation of mucociliary clearance. Trpm5-deficient mice were more susceptible to infection with a natural pathogen, and formylated bacterial peptides were detected in patients with chronic obstructive pulmonary disease. Optogenetics and peptide stimulation revealed that ciliary beating was driven by paracrine cholinergic signaling from chemosensory to ciliated cells operating through muscarinic M3 receptors independently of nerves. We provide a cellular and molecular framework that defines how tracheal chemosensory cells integrate chemosensation with innate defense.
中文翻译:

化学感应细胞衍生的乙酰胆碱响应与毒性相关的甲酰基肽而驱动气管粘膜纤毛清除。
通过协调性纤毛搏动清除粘膜纤毛是清除下呼吸道病原体的主要先天防御,但仍不清楚病原体的传感和下游信号传导机制。我们确定了毒性相关的甲酰化细菌肽,可有效刺激小鼠气管中纤毛驱动的转运。这种先天应答与甲酰基肽和味觉受体无关,但取决于关键的味觉转导基因。气管胆碱能化学感觉细胞表达了这些基因,而这些细胞的遗传消融废除了肽驱动的对粘膜纤毛清除的刺激。缺乏Trpm5的小鼠更容易感染天然病原体,并且在患有慢性阻塞性肺病的患者中检测到甲酰化细菌肽。光遗传学和肽刺激显示,纤毛跳动是由旁分泌胆碱能信号驱动的,从化学感觉到通过毒蕈碱型M3受体独立于神经运作的纤毛细胞。我们提供了一个细胞和分子框架,该框架定义了气管化学传感细胞如何将化学传感与先天防御相结合。
更新日期:2020-04-21
中文翻译:

化学感应细胞衍生的乙酰胆碱响应与毒性相关的甲酰基肽而驱动气管粘膜纤毛清除。
通过协调性纤毛搏动清除粘膜纤毛是清除下呼吸道病原体的主要先天防御,但仍不清楚病原体的传感和下游信号传导机制。我们确定了毒性相关的甲酰化细菌肽,可有效刺激小鼠气管中纤毛驱动的转运。这种先天应答与甲酰基肽和味觉受体无关,但取决于关键的味觉转导基因。气管胆碱能化学感觉细胞表达了这些基因,而这些细胞的遗传消融废除了肽驱动的对粘膜纤毛清除的刺激。缺乏Trpm5的小鼠更容易感染天然病原体,并且在患有慢性阻塞性肺病的患者中检测到甲酰化细菌肽。光遗传学和肽刺激显示,纤毛跳动是由旁分泌胆碱能信号驱动的,从化学感觉到通过毒蕈碱型M3受体独立于神经运作的纤毛细胞。我们提供了一个细胞和分子框架,该框架定义了气管化学传感细胞如何将化学传感与先天防御相结合。









































 京公网安备 11010802027423号
京公网安备 11010802027423号