当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating Process Variables and Additive Selection To Optimize Polymorphic Control of Carbamazepine in a CO2 Antisolvent Crystallization Process
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-04-13 , DOI: 10.1021/acs.oprd.9b00545 Barry Long 1 , Kevin M. Ryan 1 , Luis Padrela 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-04-13 , DOI: 10.1021/acs.oprd.9b00545 Barry Long 1 , Kevin M. Ryan 1 , Luis Padrela 1
Affiliation
|
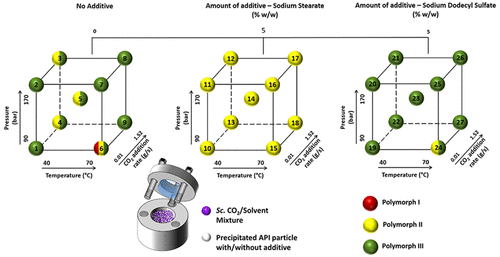
Supercritical CO2 antisolvent crystallization typically promotes the formation of metastable polymorphic forms of pharmaceutical drugs. However, using this technological approach in combination with the use of distinct molecular additives can provide further control over the final polymorphic form obtained. The work presented herein investigates the influence of critical processing variables of a CO2 antisolvent crystallization process in the presence of molecular additives with respect to the polymorphism of carbamazepine (CBZ), a highly polymorphic BCS class II drug. A Design of Experiments (DoE) approach was performed to assess the outcome of CBZ polymorphism, impacted by CO2 antisolvent processing variables such as pressure, temperature, and CO2 addition rate when anionic additives (sodium stearate or sodium dodecyl sulfate) were selected. Statistical analysis revealed that the combination of temperature and CO2 addition rate show statistically significant impact (p < 0.05) on the final CBZ polymorphic form obtained when no additive was present during short hold studies. However, when using 5% w/w additive in the CBZ methanol solutions, CBZ samples produced from CO2 antisolvent crystallization correspond to form II (when using sodium stearate as the additive) or form III (when using sodium dodecyl sulfate as the additive) for most samples, regardless of the processing conditions used. An investigation into the polymorphic stability of these CBZ samples was undertaken, allowing the precipitated CBZ to remain immersed in the supercritical media (supercritical CO2 and methanol) for a prolonged period (60 h). Carbamazepine samples that were initially form II began to convert to the stable form III at lower temperature (40 °C), while samples that were initially form III showed almost no conversion.
中文翻译:

研究工艺变量和添加剂选择以优化卡马西平在CO 2反溶剂结晶过程中的多晶型控制
超临界CO 2反溶剂结晶通常会促进药物的亚稳态多晶型形式的形成。但是,结合使用这种技术方法和使用独特的分子添加剂可以进一步控制最终获得的多晶型形式。本文介绍的工作研究了在存在分子添加剂的情况下CO 2反溶剂结晶过程的关键工艺变量对卡马西平(CBZ)(一种高度多态的BCS II类药物)的多态性的影响。进行了实验设计(DoE)方法以评估CBZ多态性的结果,该结果受CO 2反溶剂工艺变量(例如压力,温度和CO)的影响选择阴离子添加剂(硬脂酸钠或十二烷基硫酸钠)时的添加速度为2。统计分析表明,温度和CO 2的添加量的组合对在短期保存研究中不存在添加剂的情况下,最终获得的CBZ多晶型形式具有统计学显着影响(p <0.05)。但是,在CBZ甲醇溶液中使用5%w / w添加剂时,CO 2产生的CBZ样品对于大多数样品,无论使用何种加工条件,抗溶剂结晶均对应于晶型II(使用硬脂酸钠作为添加剂时)或晶型III(使用十二烷基硫酸钠作为添加剂时)。对这些CBZ样品的多晶型稳定性进行了研究,使沉淀的CBZ可以长时间(60小时)浸没在超临界介质(超临界CO 2和甲醇)中。最初为II型的卡马西平样品在较低的温度(40°C)下开始转变为稳定的III型,而最初为III型的样品几乎没有转化。
更新日期:2020-06-19
中文翻译:

研究工艺变量和添加剂选择以优化卡马西平在CO 2反溶剂结晶过程中的多晶型控制
超临界CO 2反溶剂结晶通常会促进药物的亚稳态多晶型形式的形成。但是,结合使用这种技术方法和使用独特的分子添加剂可以进一步控制最终获得的多晶型形式。本文介绍的工作研究了在存在分子添加剂的情况下CO 2反溶剂结晶过程的关键工艺变量对卡马西平(CBZ)(一种高度多态的BCS II类药物)的多态性的影响。进行了实验设计(DoE)方法以评估CBZ多态性的结果,该结果受CO 2反溶剂工艺变量(例如压力,温度和CO)的影响选择阴离子添加剂(硬脂酸钠或十二烷基硫酸钠)时的添加速度为2。统计分析表明,温度和CO 2的添加量的组合对在短期保存研究中不存在添加剂的情况下,最终获得的CBZ多晶型形式具有统计学显着影响(p <0.05)。但是,在CBZ甲醇溶液中使用5%w / w添加剂时,CO 2产生的CBZ样品对于大多数样品,无论使用何种加工条件,抗溶剂结晶均对应于晶型II(使用硬脂酸钠作为添加剂时)或晶型III(使用十二烷基硫酸钠作为添加剂时)。对这些CBZ样品的多晶型稳定性进行了研究,使沉淀的CBZ可以长时间(60小时)浸没在超临界介质(超临界CO 2和甲醇)中。最初为II型的卡马西平样品在较低的温度(40°C)下开始转变为稳定的III型,而最初为III型的样品几乎没有转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号