当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convenient synthesis of thioamidated peptides and proteins
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2020-03-22 , DOI: 10.1002/psc.3248 Bhavesh Khatri 1 , Prabhat Bhat 1 , Jayanta Chatterjee 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2020-03-22 , DOI: 10.1002/psc.3248 Bhavesh Khatri 1 , Prabhat Bhat 1 , Jayanta Chatterjee 1
Affiliation
|
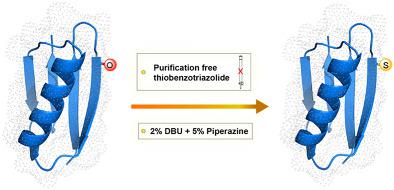
The unique physicochemical properties of a thioamide bond, which is an ideal isostere of an amide bond, have not been fully exploited because of the tedious synthesis of thionated amino acid building blocks. Here, we report a purification‐free and highly efficient synthesis of thiobenzotriazolides of Fmoc‐protected and orthogonally protected 20 naturally occurring amino acids including asparagine, glutamine, and histidine. The near‐quantitative conversion to the respective thioamidated peptides on solid support demonstrates the robustness of the synthetic route. Furthermore, the unaltered incorporation efficiency of thiobenzotriazolides from their stock solution till 48 h suggests their compatibility toward automated peptide synthesis. Finally, utilizing an optimized cocktail of 2% DBU + 5% piperazine for fast Fmoc‐deprotection, we report the synthesis of a thioamidated Pin1 WW domain and thioamidated GB1 directly on solid support.
中文翻译:

硫酰胺化肽和蛋白质的便捷合成
硫酰胺键是酰胺键的理想等排体,其独特的理化性质由于硫代氨基酸结构单元的繁琐合成而尚未得到充分利用。在这里,我们报告了由Fmoc保护和正交保护的20种天然存在的氨基酸(包括天冬酰胺,谷氨酰胺和组氨酸)的无纯化高效合成硫代苯并三唑化物。在固相支持物上将近定量转化为相应的硫代酰胺化肽表明了合成路线的稳健性。此外,硫代苯并三唑化物从其储备溶液到48小时的掺入效率未改变,表明它们与自动化肽合成具有相容性。最后,利用优化的2%DBU + 5%哌嗪混合物进行快速Fmoc脱保护,
更新日期:2020-03-22
中文翻译:

硫酰胺化肽和蛋白质的便捷合成
硫酰胺键是酰胺键的理想等排体,其独特的理化性质由于硫代氨基酸结构单元的繁琐合成而尚未得到充分利用。在这里,我们报告了由Fmoc保护和正交保护的20种天然存在的氨基酸(包括天冬酰胺,谷氨酰胺和组氨酸)的无纯化高效合成硫代苯并三唑化物。在固相支持物上将近定量转化为相应的硫代酰胺化肽表明了合成路线的稳健性。此外,硫代苯并三唑化物从其储备溶液到48小时的掺入效率未改变,表明它们与自动化肽合成具有相容性。最后,利用优化的2%DBU + 5%哌嗪混合物进行快速Fmoc脱保护,











































 京公网安备 11010802027423号
京公网安备 11010802027423号