Nature Microbiology ( IF 20.5 ) Pub Date : 2020-04-13 , DOI: 10.1038/s41564-020-0702-4 Heledd Davies 1 , Hugo Belda 1 , Malgorzata Broncel 1 , Xingda Ye 1, 2 , Claudine Bisson 3 , Viola Introini 4 , Dominique Dorin-Semblat 5, 6, 7 , Jean-Philippe Semblat 5, 6, 7 , Marta Tibúrcio 1 , Benoit Gamain 5, 6, 7 , Myrsini Kaforou 2 , Moritz Treeck 1
|
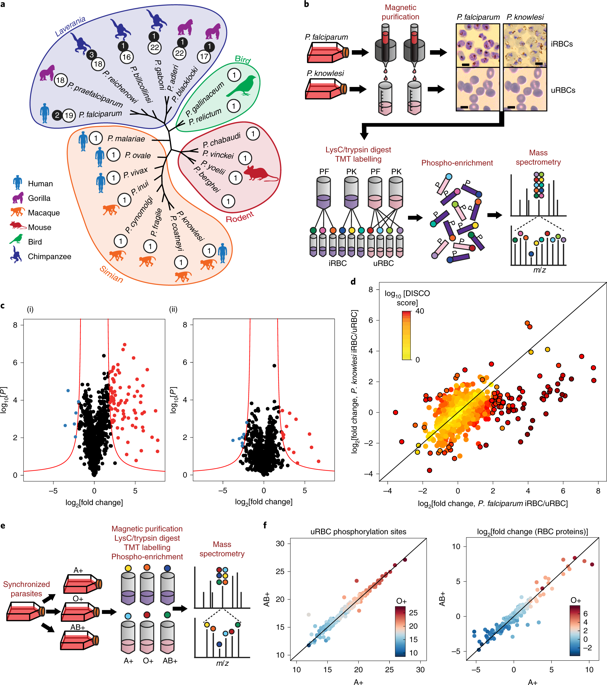
The most severe form of human malaria is caused by Plasmodium falciparum. Its virulence is closely linked to the increase in rigidity of infected erythrocytes and their adhesion to endothelial receptors, obstructing blood flow to vital organs. Unlike other human-infecting Plasmodium species, P. falciparum exports a family of 18 FIKK serine/threonine kinases into the host cell, suggesting that phosphorylation may modulate erythrocyte modifications. We reveal substantial species-specific phosphorylation of erythrocyte proteins by P. falciparum but not by Plasmodium knowlesi, which does not export FIKK kinases. By conditionally deleting all FIKK kinases combined with large-scale quantitative phosphoproteomics we identified unique phosphorylation fingerprints for each kinase, including phosphosites on parasite virulence factors and host erythrocyte proteins. Despite their non-overlapping target sites, a network analysis revealed that some FIKKs may act in the same pathways. Only the deletion of the non-exported kinase FIKK8 resulted in reduced parasite growth, suggesting the exported FIKKs may instead support functions important for survival in the host. We show that one kinase, FIKK4.1, mediates both rigidification of the erythrocyte cytoskeleton and trafficking of the adhesin and key virulence factor PfEMP1 to the host cell surface. This establishes the FIKK family as important drivers of parasite evolution and malaria pathology.
中文翻译:

输出激酶家族介导人类疟疾中物种特异性红细胞重塑和毒力。
最严重的人类疟疾是由恶性疟原虫引起的。其毒力与受感染红细胞硬度的增加及其与内皮受体的粘附密切相关,从而阻碍血液流向重要器官。与其他感染人类的疟原虫物种不同,恶性疟原虫将 18 种 FIKK 丝氨酸/苏氨酸激酶家族输出到宿主细胞中,这表明磷酸化可能会调节红细胞修饰。我们揭示了恶性疟原虫对红细胞蛋白的大量物种特异性磷酸化,但诺氏疟原虫却没有,后者不输出 FIKK 激酶。通过有条件地删除所有 FIKK 激酶并结合大规模定量磷酸化蛋白质组学,我们确定了每种激酶的独特磷酸化指纹,包括寄生虫毒力因子和宿主红细胞蛋白上的磷酸位点。尽管它们的目标位点不重叠,但网络分析显示,一些 FIKK 可能以相同的途径发挥作用。只有删除非输出激酶 FIKK8 才会导致寄生虫生长减少,这表明输出的 FIKK 可能反而支持对宿主生存至关重要的功能。我们发现,一种激酶 FIKK4.1 介导红细胞细胞骨架的硬化以及粘附素和关键毒力因子 PfEMP1 向宿主细胞表面的运输。这使得 FIKK 家族成为寄生虫进化和疟疾病理学的重要驱动因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号