当前位置:
X-MOL 学术
›
Comput. Biol. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tissue-specific and interpretable sub-segmentation of whole tumour burden on CT images by unsupervised fuzzy clustering.
Computers in Biology and Medicine ( IF 7.0 ) Pub Date : 2020-04-10 , DOI: 10.1016/j.compbiomed.2020.103751 Leonardo Rundo 1 , Lucian Beer 2 , Stephan Ursprung 1 , Paula Martin-Gonzalez 3 , Florian Markowetz 3 , James D Brenton 3 , Mireia Crispin-Ortuzar 3 , Evis Sala 1 , Ramona Woitek 2
Computers in Biology and Medicine ( IF 7.0 ) Pub Date : 2020-04-10 , DOI: 10.1016/j.compbiomed.2020.103751 Leonardo Rundo 1 , Lucian Beer 2 , Stephan Ursprung 1 , Paula Martin-Gonzalez 3 , Florian Markowetz 3 , James D Brenton 3 , Mireia Crispin-Ortuzar 3 , Evis Sala 1 , Ramona Woitek 2
Affiliation
|
BACKGROUND
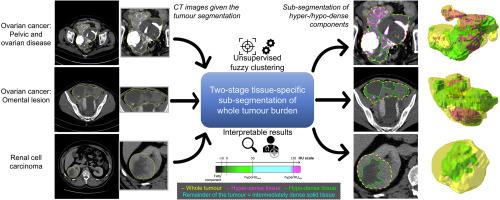
Cancer typically exhibits genotypic and phenotypic heterogeneity, which can have prognostic significance and influence therapy response. Computed Tomography (CT)-based radiomic approaches calculate quantitative features of tumour heterogeneity at a mesoscopic level, regardless of macroscopic areas of hypo-dense (i.e., cystic/necrotic), hyper-dense (i.e., calcified), or intermediately dense (i.e., soft tissue) portions.
METHOD
With the goal of achieving the automated sub-segmentation of these three tissue types, we present here a two-stage computational framework based on unsupervised Fuzzy C-Means Clustering (FCM) techniques. No existing approach has specifically addressed this task so far. Our tissue-specific image sub-segmentation was tested on ovarian cancer (pelvic/ovarian and omental disease) and renal cell carcinoma CT datasets using both overlap-based and distance-based metrics for evaluation.
RESULTS
On all tested sub-segmentation tasks, our two-stage segmentation approach outperformed conventional segmentation techniques: fixed multi-thresholding, the Otsu method, and automatic cluster number selection heuristics for the K-means clustering algorithm. In addition, experiments showed that the integration of the spatial information into the FCM algorithm generally achieves more accurate segmentation results, whilst the kernelised FCM versions are not beneficial. The best spatial FCM configuration achieved average Dice similarity coefficient values starting from 81.94±4.76 and 83.43±3.81 for hyper-dense and hypo-dense components, respectively, for the investigated sub-segmentation tasks.
CONCLUSIONS
The proposed intelligent framework could be readily integrated into clinical research environments and provides robust tools for future radiomic biomarker validation.
中文翻译:

通过无监督模糊聚类对 CT 图像上的整个肿瘤负荷进行组织特异性和可解释的细分。
背景技术癌症通常表现出基因型和表型异质性,这可能具有预后意义并影响治疗反应。基于计算机断层扫描 (CT) 的放射组学方法可在介观水平上计算肿瘤异质性的定量特征,而不管宏观区域的低密度(即囊性/坏死)、高密度(即钙化)或中等密度(即,软组织)部分。方法 为了实现这三种组织类型的自动细分,我们在此提出了一个基于无监督模糊 C 均值聚类 (FCM) 技术的两阶段计算框架。到目前为止,还没有任何现有方法专门解决此任务。我们的组织特异性图像子分割在卵巢癌(盆腔/卵巢和网膜疾病)和肾细胞癌 CT 数据集上进行了测试,使用基于重叠和基于距离的指标进行评估。结果在所有测试的子分割任务中,我们的两阶段分割方法优于传统分割技术:固定多阈值、Otsu 方法和 K 均值聚类算法的自动聚类数选择启发式。此外,实验表明,将空间信息集成到 FCM 算法中通常可以获得更准确的分割结果,而内核化的 FCM 版本则没有什么好处。对于所研究的子分割任务,最佳空间 FCM 配置的高密度和低密度组件的平均 Dice 相似系数值分别为 81.94±4.76 和 83.43±3.81。 结论所提出的智能框架可以很容易地集成到临床研究环境中,并为未来的放射组学生物标志物验证提供强大的工具。
更新日期:2020-04-21
中文翻译:

通过无监督模糊聚类对 CT 图像上的整个肿瘤负荷进行组织特异性和可解释的细分。
背景技术癌症通常表现出基因型和表型异质性,这可能具有预后意义并影响治疗反应。基于计算机断层扫描 (CT) 的放射组学方法可在介观水平上计算肿瘤异质性的定量特征,而不管宏观区域的低密度(即囊性/坏死)、高密度(即钙化)或中等密度(即,软组织)部分。方法 为了实现这三种组织类型的自动细分,我们在此提出了一个基于无监督模糊 C 均值聚类 (FCM) 技术的两阶段计算框架。到目前为止,还没有任何现有方法专门解决此任务。我们的组织特异性图像子分割在卵巢癌(盆腔/卵巢和网膜疾病)和肾细胞癌 CT 数据集上进行了测试,使用基于重叠和基于距离的指标进行评估。结果在所有测试的子分割任务中,我们的两阶段分割方法优于传统分割技术:固定多阈值、Otsu 方法和 K 均值聚类算法的自动聚类数选择启发式。此外,实验表明,将空间信息集成到 FCM 算法中通常可以获得更准确的分割结果,而内核化的 FCM 版本则没有什么好处。对于所研究的子分割任务,最佳空间 FCM 配置的高密度和低密度组件的平均 Dice 相似系数值分别为 81.94±4.76 和 83.43±3.81。 结论所提出的智能框架可以很容易地集成到临床研究环境中,并为未来的放射组学生物标志物验证提供强大的工具。









































 京公网安备 11010802027423号
京公网安备 11010802027423号