Geoscience Frontiers ( IF 8.5 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.gsf.2020.03.010 Zifan Wang , Haixu Cui , Jingyu Hou , Xiao Dong
|
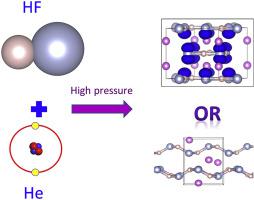
HHe+ is considered as the strongest acid and most powerful proton donor known to human. Whether HHe+ exists at planetary high pressure environment is a quite important problem in physics, chemistry and planetary sciences. Here, using the ab initio evolutionary algorithm USPEX package, we searched HF–He system, which was reported as the most possible candidate to contain HHe+. The calculation proved HHe+ cannot form at pressure <1000 GPa, due to a conflict between the covalent component in symmetric hydrogen bond and ionic HHe+. Although He atoms have no chemical bonding with other elements, they can supply a chemical pressure, leading to two new phases He2(HF)4 and He(HF). With coplanar (HF)4 rings, He2(HF)4 have an aromaticity-like electronic behavior while He(HF) has a new type of chiral HF chain. The formation of He2(HF)4 and He(HF) prove that the chemical pressure from He, on par with external pressure, have ability to control the structural and electronic configuration and induce some new familiars of compounds include H and He elements which are fundamental planetary materials in giant planets.
中文翻译:

HHe +能否在高压下存在:高压诱导的HF-He化合物的探索
HHe +被认为是人类已知的最强酸和最强大的质子供体。HHe +是否存在于行星高压环境中是物理,化学和行星科学中一个非常重要的问题。在这里,我们使用从头算进化算法USPEX包,搜索了HF-He系统,该系统被报告为最可能包含HHe +的候选物。计算证明,由于对称氢键中的共价成分与离子性HHe +之间存在冲突,HHe +不能在<1000 GPa的压力下形成。尽管He原子与其他元素没有化学键合,但它们可以提供化学压力,从而导致两个新的相He 2(HF)4和He(HF)。通过共面(HF)4环,He 2(HF)4具有类似芳香性的电子行为,而He(HF)具有新型手性HF链。He 2(HF)4和He(HF)的形成证明,来自He的化学压力与外部压力相同,具有控制结构和电子构型的能力,并诱导了一些新的熟悉的化合物,包括H和He元素,这些元素是巨型行星的基本行星材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号