当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia, and withdrawal in mice
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2020-04-06 , DOI: 10.1002/jnr.24626 David Reiss 1, 2, 3, 4 , Tando Maduna 1, 2, 3, 4, 5 , Hervé Maurin 1, 2, 3, 4 , Emilie Audouard 1, 2, 3, 4 , Claire Gaveriaux-Ruff 1, 2, 3, 4, 6
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2020-04-06 , DOI: 10.1002/jnr.24626 David Reiss 1, 2, 3, 4 , Tando Maduna 1, 2, 3, 4, 5 , Hervé Maurin 1, 2, 3, 4 , Emilie Audouard 1, 2, 3, 4 , Claire Gaveriaux-Ruff 1, 2, 3, 4, 6
Affiliation
|
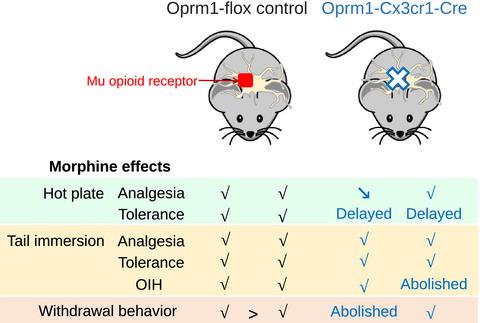
A major challenge in medicine is developing potent pain therapies without the adverse effects of opiates. Neuroinflammation and in particular microglial activation have been shown to contribute to these effects. However, the implication of the microglial mu opioid receptor (MOR) is not known. We developed a novel conditional knockout (cKO) mouse line, wherein MOR is deleted in microglia. Morphine analgesic tolerance was delayed in both sexes in cKO mice in the hot plate assay. Opioid-induced hyperalgesia (OIH) as measured in the tail immersion assay was abolished in male cKO mice, and physical dependence to morphine as assessed by naloxone-induced withdrawal was attenuated in female cKO mice. Our results show a sex-dependent contribution of microglial MOR in morphine analgesic tolerance, OIH, and physical dependence. In conclusion, our data suggest that blockade of microglial MOR could represent a therapeutic target for opiate analgesia without the opiate adverse effects.
中文翻译:

小胶质细胞中的 Mu 阿片受体有助于小鼠的吗啡镇痛耐受、痛觉过敏和戒断
医学上的一个主要挑战是开发没有阿片类药物副作用的有效疼痛疗法。神经炎症,特别是小胶质细胞激活已被证明有助于这些影响。然而,小胶质细胞阿片受体 (MOR) 的含义尚不清楚。我们开发了一种新的条件性敲除 (cKO) 小鼠品系,其中 MOR 在小胶质细胞中被删除。在热板试验中,cKO 小鼠的两性均延迟了吗啡镇痛耐受性。在雄性 cKO 小鼠中消除了在尾部浸泡试验中测量的阿片类药物诱导的痛觉过敏 (OIH),并且在雌性 cKO 小鼠中,通过纳洛酮诱导的戒断评估对吗啡的身体依赖性减弱。我们的研究结果表明,小胶质细胞 MOR 在吗啡镇痛耐受性、OIH 和身体依赖性方面具有性别依赖性。综上所述,
更新日期:2020-04-06
中文翻译:

小胶质细胞中的 Mu 阿片受体有助于小鼠的吗啡镇痛耐受、痛觉过敏和戒断
医学上的一个主要挑战是开发没有阿片类药物副作用的有效疼痛疗法。神经炎症,特别是小胶质细胞激活已被证明有助于这些影响。然而,小胶质细胞阿片受体 (MOR) 的含义尚不清楚。我们开发了一种新的条件性敲除 (cKO) 小鼠品系,其中 MOR 在小胶质细胞中被删除。在热板试验中,cKO 小鼠的两性均延迟了吗啡镇痛耐受性。在雄性 cKO 小鼠中消除了在尾部浸泡试验中测量的阿片类药物诱导的痛觉过敏 (OIH),并且在雌性 cKO 小鼠中,通过纳洛酮诱导的戒断评估对吗啡的身体依赖性减弱。我们的研究结果表明,小胶质细胞 MOR 在吗啡镇痛耐受性、OIH 和身体依赖性方面具有性别依赖性。综上所述,











































 京公网安备 11010802027423号
京公网安备 11010802027423号