当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antimicrobial Pyridazines: Synthesis, Characterization, Cytotoxicity, Promiscuity, and Molecular Docking
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-05-08 , DOI: 10.1002/cbdv.202000100 Muhamad Mustafa 1 , Yaser A Mostafa 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-05-08 , DOI: 10.1002/cbdv.202000100 Muhamad Mustafa 1 , Yaser A Mostafa 2
Affiliation
|
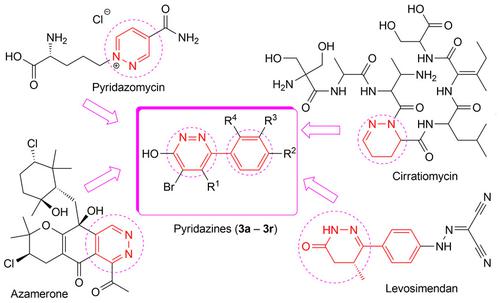
A facile and convenient synthesis of new pyridazines suitable for use as antimicrobial agents was reported. The hydrazide intermediate was coupled with various benzaldehydes and/or acetophenones and cyclized instantaneously to afford target pyridazine derivatives. The structures of new pyridazines were confirmed by IR, 1H‐ and 13C‐NMR, elemental analysis in addition to representative LC/MS. Antimicrobial activity was screened against 10 bacterial and fungal strains. The new pyridazines showed strong to very strong antibacterial activity against Gram‐negative (GNB) bacteria, while none of them showed significant antifungal activity at the same concentration range. Chloro derivatives exhibited the highest antibacterial activity with MICs (0.892–3.744 μg/mL) lower than that of chloramphenicol (2.019–8.078 μg/mL) against E. coli, P. aeruginosa, and S. marcescens. Prediction of ADME parameters, pharmacokinetics, and substrate promiscuity revealed that these new pyridazines could be promising drug candidates. Cytotoxic studies on rat hepatocytes showed how much safe these new pyridazines on living organisms (IC50>64 μg/mL). MOE docking studies showed a good overlay of these new pyridazines with co‐crystallized ligand within an E. coli DNA gyrase subunit B active sites (4KFG).
中文翻译:

抗菌哒嗪:合成、表征、细胞毒性、混杂和分子对接
报道了适合用作抗微生物剂的新型哒嗪的简便合成。酰肼中间体与各种苯甲醛和/或苯乙酮偶联并立即环化以提供目标哒嗪衍生物。除了具有代表性的 LC/MS 外,还通过 IR、1H 和 13C-NMR、元素分析证实了新哒嗪的结构。针对 10 种细菌和真菌菌株筛选了抗菌活性。新型哒嗪对革兰氏阴性(GNB)细菌显示出很强的抗菌活性,而在相同浓度范围内,它们都没有显示出显着的抗真菌活性。氯衍生物表现出最高的抗菌活性,MIC(0.892–3.744 μg/mL)低于氯霉素(2.019–8.078 μg/mL)对大肠杆菌、铜绿假单胞菌和金黄色葡萄球菌的抗菌活性。粘质。ADME 参数、药代动力学和底物混杂的预测表明,这些新的哒嗪可能是有希望的候选药物。对大鼠肝细胞的细胞毒性研究表明这些新型哒嗪对生物体的安全性(IC50>64 μg/mL)。MOE 对接研究表明,这些新型哒嗪与大肠杆菌 DNA 促旋酶 B 亚基活性位点 (4KFG) 内的共结晶配体有良好的重叠。
更新日期:2020-05-08
中文翻译:

抗菌哒嗪:合成、表征、细胞毒性、混杂和分子对接
报道了适合用作抗微生物剂的新型哒嗪的简便合成。酰肼中间体与各种苯甲醛和/或苯乙酮偶联并立即环化以提供目标哒嗪衍生物。除了具有代表性的 LC/MS 外,还通过 IR、1H 和 13C-NMR、元素分析证实了新哒嗪的结构。针对 10 种细菌和真菌菌株筛选了抗菌活性。新型哒嗪对革兰氏阴性(GNB)细菌显示出很强的抗菌活性,而在相同浓度范围内,它们都没有显示出显着的抗真菌活性。氯衍生物表现出最高的抗菌活性,MIC(0.892–3.744 μg/mL)低于氯霉素(2.019–8.078 μg/mL)对大肠杆菌、铜绿假单胞菌和金黄色葡萄球菌的抗菌活性。粘质。ADME 参数、药代动力学和底物混杂的预测表明,这些新的哒嗪可能是有希望的候选药物。对大鼠肝细胞的细胞毒性研究表明这些新型哒嗪对生物体的安全性(IC50>64 μg/mL)。MOE 对接研究表明,这些新型哒嗪与大肠杆菌 DNA 促旋酶 B 亚基活性位点 (4KFG) 内的共结晶配体有良好的重叠。











































 京公网安备 11010802027423号
京公网安备 11010802027423号