当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen sulfide increases copper-dependent neurotoxicity via intracellular copper accumulation.
Metallomics ( IF 2.9 ) Pub Date : 2020-04-10 , DOI: 10.1039/d0mt00015a Norika Goto 1 , Hirokazu Hara 1 , Mao Kondo 1 , Naomi Yasuda 1 , Tetsuro Kamiya 1 , Kensuke Okuda 2 , Tetsuo Adachi 1
Metallomics ( IF 2.9 ) Pub Date : 2020-04-10 , DOI: 10.1039/d0mt00015a Norika Goto 1 , Hirokazu Hara 1 , Mao Kondo 1 , Naomi Yasuda 1 , Tetsuro Kamiya 1 , Kensuke Okuda 2 , Tetsuo Adachi 1
Affiliation
|
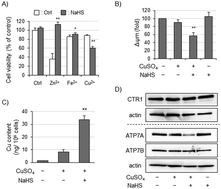
Copper (Cu) is an essential trace element and acts as a redox cofactor for many enzymes; however, excess Cu is toxic to cells. Hydrogen sulfide (H2S) is a well-known toxic gaseous molecule, but it has various biological effects such as neuromodulation and vasodilation. H2S was recently demonstrated to be involved in the detoxification of heavy metals, including zinc and cadmium, suggesting that H2S helps to maintain the homeostasis of heavy metals in cells. However, it is unclear how H2S impacts cellular Cu dynamics. In this study, we examined the effects of H2S on Cu cytotoxicity. Human neuroblastoma SH-SY5Y cells were exposed to CuSO4 in the presence of the H2S donor NaHS. CuSO4 alone slightly induced cell injury, whereas the combination of CuSO4 and NaHS (Cu/NaHS) increased Cu cytotoxicity. The Cu chelator bathocuproinedisulfonic acid mitigated Cu/NaHS-induced cytotoxicity. Compared with CuSO4 alone, Cu/NaHS markedly promoted ROS generation, mitochondrial dysfunction, and a decrease in ATP production. In addition, reporter assay using the metal responsive element (MRE)-driven reporter plasmid revealed that Cu/NaHS augmented Cu-dependent MRE activation. The amount of intracellular Cu was significantly higher in cells treated with Cu/NaHS than in those treated with CuSO4 alone. Moreover, Cu/NaHS markedly suppressed the level of the Cu exporter ATP7A, but not ATP7B, protein, whereas the combination did not affect that of the Cu importer CTR1 protein. Taken together, we conclude that the marked decrease in the ATP7A protein level by Cu/NaHS promotes intracellular Cu accumulation and leads to increased Cu cytotoxicity.
中文翻译:

硫化氢通过细胞内铜积累增加铜依赖性神经毒性。
铜 (Cu) 是一种必需的微量元素,是许多酶的氧化还原辅因子;然而,过量的铜对细胞是有毒的。硫化氢 (H 2 S) 是一种众所周知的有毒气体分子,但它具有多种生物效应,例如神经调节和血管舒张。最近证明H 2 S 参与重金属(包括锌和镉)的解毒,这表明 H 2 S 有助于维持细胞中重金属的稳态。然而,尚不清楚 H 2 S如何影响细胞 Cu 动力学。在这项研究中,我们检查了 H 2 S 对 Cu 细胞毒性的影响。在 H 2存在下将人神经母细胞瘤 SH-SY5Y 细胞暴露于 CuSO 4S 供体 NaHS。CuSO 4单独轻微诱导细胞损伤,而CuSO 4和NaHS (Cu/NaHS)的组合增加Cu细胞毒性。Cu 螯合剂浴铜灵二磺酸减轻了 Cu/NaHS 诱导的细胞毒性。与单独的CuSO 4相比,Cu/NaHS 显着促进 ROS 生成、线粒体功能障碍和 ATP 生成减少。此外,使用金属响应元件 (MRE) 驱动的报告质粒的报告基因检测显示,Cu/NaHS 增强了 Cu 依赖性 MRE 激活。Cu/NaHS处理的细胞内Cu含量显着高于CuSO 4处理的细胞独自的。此外,Cu/NaHS 显着抑制了 Cu 出口蛋白 ATP7A 的水平,但不影响 ATP7B 蛋白的水平,而这种组合不影响 Cu 进口蛋白 CTR1 蛋白的水平。综上所述,我们得出结论,Cu/NaHS 引起的 ATP7A 蛋白水平的显着降低促进细胞内 Cu 积累并导致 Cu 细胞毒性增加。
更新日期:2020-04-10
中文翻译:

硫化氢通过细胞内铜积累增加铜依赖性神经毒性。
铜 (Cu) 是一种必需的微量元素,是许多酶的氧化还原辅因子;然而,过量的铜对细胞是有毒的。硫化氢 (H 2 S) 是一种众所周知的有毒气体分子,但它具有多种生物效应,例如神经调节和血管舒张。最近证明H 2 S 参与重金属(包括锌和镉)的解毒,这表明 H 2 S 有助于维持细胞中重金属的稳态。然而,尚不清楚 H 2 S如何影响细胞 Cu 动力学。在这项研究中,我们检查了 H 2 S 对 Cu 细胞毒性的影响。在 H 2存在下将人神经母细胞瘤 SH-SY5Y 细胞暴露于 CuSO 4S 供体 NaHS。CuSO 4单独轻微诱导细胞损伤,而CuSO 4和NaHS (Cu/NaHS)的组合增加Cu细胞毒性。Cu 螯合剂浴铜灵二磺酸减轻了 Cu/NaHS 诱导的细胞毒性。与单独的CuSO 4相比,Cu/NaHS 显着促进 ROS 生成、线粒体功能障碍和 ATP 生成减少。此外,使用金属响应元件 (MRE) 驱动的报告质粒的报告基因检测显示,Cu/NaHS 增强了 Cu 依赖性 MRE 激活。Cu/NaHS处理的细胞内Cu含量显着高于CuSO 4处理的细胞独自的。此外,Cu/NaHS 显着抑制了 Cu 出口蛋白 ATP7A 的水平,但不影响 ATP7B 蛋白的水平,而这种组合不影响 Cu 进口蛋白 CTR1 蛋白的水平。综上所述,我们得出结论,Cu/NaHS 引起的 ATP7A 蛋白水平的显着降低促进细胞内 Cu 积累并导致 Cu 细胞毒性增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号