Journal of Human Genetics ( IF 2.6 ) Pub Date : 2020-04-10 , DOI: 10.1038/s10038-020-0744-8 Nivethitha Arunkumar 1, 2 , Thomas J Langan 3 , Molly Stapleton 1, 4 , Francyne Kubaski 5, 6, 7 , Robert W Mason 1, 4 , Rajendra Singh 8 , Hironori Kobayashi 9 , Seiji Yamaguchi 9 , Yasuyuki Suzuki 10 , Kenji Orii 11 , Tadao Orii 11 , Toshiyuki Fukao 11 , Shunji Tomatsu 1, 4, 9, 11, 12
|
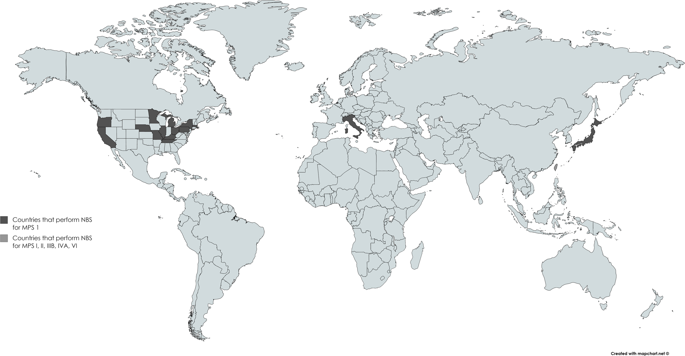
Mucopolysaccharidoses (MPS) are a subtype of lysosomal storage disorders (LSDs) characterized by the deficiency of the enzyme involved in the breakdown of glycosaminoglycans (GAGs). Mucopolysaccharidosis type I (MPS I, Hurler Syndrome) was endorsed by the U.S. Secretary of the Department of Health and Human Services for universal newborn screening (NBS) in February 2016. Its endorsement exemplifies the need to enhance the accuracy of diagnostic testing for disorders that are considered for NBS. The progression of MPS disorders typically incudes irreversible CNS involvement, severe bone dysplasia, and cardiac and respiratory issues. Patients with MPS have a significantly decreased quality of life if untreated and require timely diagnosis and management for optimal outcomes. NBS provides the opportunity to diagnose and initiate treatment plans for MPS patients as early as possible. Most newborns with MPS are asymptomatic at birth; therefore, it is crucial to have biomarkers that can be identified in the newborn. At present, there are tiered methods and different instrumentation available for this purpose. The screening of quick, cost-effective, sensitive, and specific biomarkers in patients with MPS at birth is important. Rapid newborn diagnosis enables treatments to maximize therapeutic efficacy and to introduce immune tolerance during the neonatal period. Currently, newborn screening for MPS I and II has been implemented and/or in pilot testing in several countries. In this review article, historical aspects of NBS for MPS and the prospect of newborn screening for MPS are described, including the potential tiers of screening.
中文翻译:

新生儿粘多糖贮积症筛查:过去、现在和未来。
粘多糖贮积症 (MPS) 是溶酶体贮积症 (LSD) 的一种亚型,其特征是缺乏参与糖胺聚糖 (GAG) 分解的酶。粘多糖贮积症 I 型(MPS I,Hurler 综合症)于 2016 年 2 月获得美国卫生与公共服务部部长的认可,用于新生儿普查 (NBS)。它的认可表明需要提高诊断测试的准确性被考虑用于国家统计局。MPS 疾病的进展通常包括不可逆的 CNS 受累、严重的骨发育不良以及心脏和呼吸系统问题。如果不治疗,MPS 患者的生活质量会显着下降,需要及时诊断和管理以获得最佳结果。NBS 为 MPS 患者提供了尽早诊断和启动治疗计划的机会。大多数患有 MPS 的新生儿在出生时没有症状;因此,拥有可在新生儿中识别的生物标志物至关重要。目前,有分层方法和不同的仪器可用于此目的。对出生时患有 MPS 的患者进行快速、经济、敏感和特异的生物标志物筛查非常重要。快速新生儿诊断使治疗能够最大限度地提高治疗效果,并在新生儿期引入免疫耐受。目前,MPS I 和 II 的新生儿筛查已经在几个国家实施和/或进行试点测试。在这篇评论文章中,描述了 MPS NBS 的历史方面和新生儿 MPS 筛查的前景,











































 京公网安备 11010802027423号
京公网安备 11010802027423号