Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-04-09 , DOI: 10.1016/j.jfluchem.2020.109519 Ahmed M.A. Ahmed , Adnan I. Mohammed , Roger W. Read
|
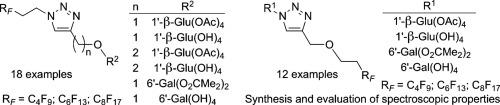
A library of 10 series of homologous compounds making up a total of 30 new, fluorous 1-substituted 1,2,3-triazol-4-ylmethyl(and ethyl) ether surfactant candidates, with highly systematic variations in substituent position and type, have been synthesised in consistently good yields by processes involving copper(I)-accelerated Huisgen-Meldal dipolar cycloaddition reactions. Each series contains a perfluorobutylethyl, perfluorohexylethyl or perfluorooctylethyl substituents at triazole position N(1) or attached through the triazole ether oxygen, and either a 1-β-D-glucosyl or 6-α-D-galactosyl substituent at the alternative triazole position. Half the library was prepared directly with the sugar components in a protected form (peracetylated glucose derivatives and 1,2:3,4-bisacetonide galactose derivatives) while the other series with unprotected sugars were obtained following a secondary deprotection step by trans-esterification using sodium methoxide or acid catalysed acetal solvation.
All the triazoles are candidates for study as fluorous surfactants that are switchable between amphiphilic partner states, hydrophobic in the case of protected sugars and hydrophilic in unprotected sugars. As a prelude to surfactant studies, the NMR spectroscopic characteristics of the newly establish library of compounds were examined. Data were compared and contrasted within and between each series with reference to related literature analogues. Analysis of unprotected 6-galactosyl derivatives was complicated by the well known generation of inseparable, epimeric mixtures of hemiacetal pyranose and furanose products and the general paucity in the literature of NMR spectroscopic data assignments of signals from 1-β-hydroxygalactopyranose compounds. These matters have been addressed in this paper and the comparisons include analyses of unprotected 1'-α- and 1'-β-hydroxy-pyranose derivatives.
中文翻译:

走向功能性氟表面活性剂。系统修饰的糖取代的氟1,2,3-三唑的合成及光谱特征
由10个系列的同源化合物组成的库,总共有30种新的氟代1-取代的1,2,3-三唑-4-甲基(和乙基)醚表面活性剂候选物,其取代基的位置和类型高度系统地变化通过涉及铜(I)加速的惠斯根-梅德尔偶极环加成反应的过程以始终如一的高收率合成了苯甲酸。每个系列在三唑位置N(1)处或通过三唑醚氧连接的全氟丁基乙基,全氟己基乙基或全氟辛基乙基取代基,以及在另一个三唑位置的1-β-D-葡萄糖基或6-α-D-半乳糖基取代基。一半的文库是直接用保护形式的糖成分(过乙酰化的葡萄糖衍生物和1,2:3,
所有的三唑都是可作为氟表面活性剂进行研究的候选物,它们可以在两亲性伙伴状态之间切换,在保护糖的情况下是疏水的,在未保护糖中是亲水的。作为表面活性剂研究的序幕,检查了新建立的化合物库的NMR光谱特征。参照相关文献类似物,对每个系列之内和之间的数据进行比较和对比。未保护的6-半乳糖基衍生物的分析由于众所周知的半缩醛吡喃糖和呋喃糖产物的不可分割的差向异构混合物的产生而变得复杂,并且从1-β-羟基吡喃半乳糖化合物发出的信号的NMR光谱数据分配的文献中普遍缺乏。











































 京公网安备 11010802027423号
京公网安备 11010802027423号