当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, biological activities, and docking study of novel chalcone-pleuromutilin derivatives.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-04-09 , DOI: 10.1111/cbdd.13692 Chuan Xie 1 , Siyu Zhang 2 , Wei Zhang 1 , Chunxia Wu 1 , Can Yong 1 , Yuhao Sun 2 , Zhengxing Zeng 1 , Qian Zhang 2 , Zixin Huang 1 , Tian Chen 2 , Yuanyuan Zhang 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-04-09 , DOI: 10.1111/cbdd.13692 Chuan Xie 1 , Siyu Zhang 2 , Wei Zhang 1 , Chunxia Wu 1 , Can Yong 1 , Yuhao Sun 2 , Zhengxing Zeng 1 , Qian Zhang 2 , Zixin Huang 1 , Tian Chen 2 , Yuanyuan Zhang 1
Affiliation
|
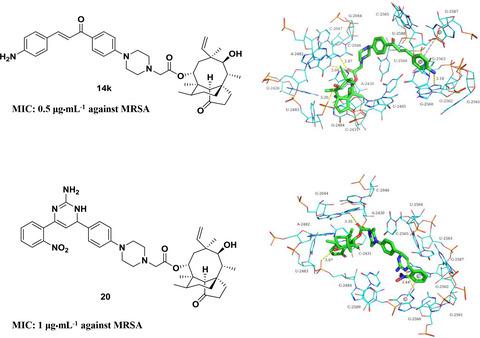
The issue of antibiotic resistance is becoming progressively serious these days, and the feasible solution to address it is to develop and discover novel antibiotics. The diterpene natural pleuromutilin is a prominent candidate for its special mode of action by inhibiting protein synthesis. In this study, a series of novel pleuromutilin derivatives with chalcone moiety was designed and synthesized, and their antibacterial activities were assessed in vitro. As suggested from the results, most of compounds exhibited potent activities against two methicillin‐resistant Staphylococcus aureus (MRSA) ATCC 33591 and 43300. The further modification of the chalcone structure, aza‐cyclic derivatives were afforded and then assessed, and potent activities against the tested strains were reported. The preliminary docking studies were conducted to explore the interactions between target molecules and binding site.
中文翻译:

新型查尔酮-截短侧耳素衍生物的合成、生物活性及对接研究。
如今,抗生素耐药性问题日益严重,解决这一问题的可行解决方案是开发和发现新型抗生素。二萜天然截短侧耳素因其抑制蛋白质合成的特殊作用模式而成为突出的候选者。本研究设计并合成了一系列具有查耳酮部分的新型截短侧耳素衍生物,并对其体外抗菌活性进行了评估。结果表明,大多数化合物对两种耐甲氧西林金黄色葡萄球菌(MRSA) ATCC 33591 和 43300 表现出有效的活性。对查尔酮结构、氮杂环衍生物进行进一步修饰,然后进行评估,发现其对 MRSA 具有有效的活性。报告了测试菌株。进行初步对接研究以探索靶分子与结合位点之间的相互作用。
更新日期:2020-04-09
中文翻译:

新型查尔酮-截短侧耳素衍生物的合成、生物活性及对接研究。
如今,抗生素耐药性问题日益严重,解决这一问题的可行解决方案是开发和发现新型抗生素。二萜天然截短侧耳素因其抑制蛋白质合成的特殊作用模式而成为突出的候选者。本研究设计并合成了一系列具有查耳酮部分的新型截短侧耳素衍生物,并对其体外抗菌活性进行了评估。结果表明,大多数化合物对两种耐甲氧西林金黄色葡萄球菌(MRSA) ATCC 33591 和 43300 表现出有效的活性。对查尔酮结构、氮杂环衍生物进行进一步修饰,然后进行评估,发现其对 MRSA 具有有效的活性。报告了测试菌株。进行初步对接研究以探索靶分子与结合位点之间的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号