当前位置:
X-MOL 学术
›
ChemNanoMat
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlled One‐pot Synthesis of Nickel Single Atoms Embedded in Carbon Nanotube and Graphene Supports with High Loading
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-04-29 , DOI: 10.1002/cnma.202000223 Shiyong Zhao 1 , Tianshuai Wang 2 , Guangmin Zhou 3 , Liji Zhang 4, 5 , Chao Lin 2 , Jean‐Pierre Veder 6 , Bernt Johannessen 7 , Martin Saunders 8 , Lichang Yin 9 , Chang Liu 9 , Roland De Marco 10, 11 , Shi‐Ze Yang 12 , Qianfan Zhang 2 , San Ping Jiang 1
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-04-29 , DOI: 10.1002/cnma.202000223 Shiyong Zhao 1 , Tianshuai Wang 2 , Guangmin Zhou 3 , Liji Zhang 4, 5 , Chao Lin 2 , Jean‐Pierre Veder 6 , Bernt Johannessen 7 , Martin Saunders 8 , Lichang Yin 9 , Chang Liu 9 , Roland De Marco 10, 11 , Shi‐Ze Yang 12 , Qianfan Zhang 2 , San Ping Jiang 1
Affiliation
|
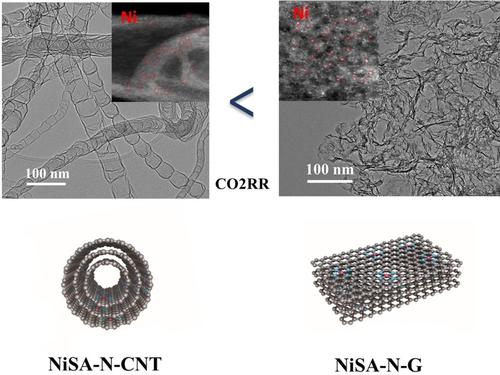
Single‐atom catalysts (SACs) have attracted much attentions due to the advantages of high catalysis efficiency and selectivity. However, the controllable and efficient synthesis of SACs remains a significant challenge. Herein, we report a controlled one‐pot synthesis of nickel single atoms embedded on nitrogen‐doped carbon nanotubes (NiSA−N−CNT) and nitrogen‐doped graphene (NiSA−N−G). The formation of NiSA−N−CNT is due to the solid‐to‐solid rolling up mechanism during the high temperature pyrolysis at 800 °C from the stacked and layered Ni‐doped g‐C3N4, g‐C3N4−Ni structure to a tubular CNT structure. Addition of citric acid introduces an amorphous carbon source on the layered g‐C3N4−Ni and after annealing at the same temperature of 800 °C, instead of formation of NiSA−N−CNT, Ni single atoms embedded in planar graphene type supports, NiSA−N−G were obtained. The density functional theory (DFT) calculation indicates the introduction of amorphous carbon source substantially reduces the structure fluctuation or curvature of layered g‐C3N4‐Ni intermediate products, thus interrupting the solid‐to‐solid rolling process and leading to the formation of planar graphene type supports for Ni single atoms. The as‐synthesized NiSA−N−G with Ni atomic loading of ∼6 wt% catalysts shows a better activity and stability for the CO2 reduction reaction (CO2RR) than NiSA−N−CNT with Ni atomic loading of ∼15 wt% due to the open and exposed Ni single atom active sites in NiSA−N−G. This study demonstrates for the first time the feasibility in the control of the microstructure of carbon supports in the synthesis of SACs.
中文翻译:

可控的一锅法合成高负载碳纳米管和石墨烯载体中嵌入的镍单原子
单原子催化剂(SAC)由于具有高催化效率和选择性的优势而备受关注。但是,SAC的可控和有效合成仍然是一个重大挑战。在本文中,我们报告了氮掺杂碳纳米管(NiSA-N-CNT)和氮掺杂石墨烯(NiSA-NG-G)上嵌入的镍单原子的受控单锅合成。NiSA-N-CNT的形成是由于在800°C的高温下热解过程中,从堆积和分层的掺杂Ni的g-C 3 N 4,g-C 3 N 4的固-固卷积机制引起的-Ni结构为管状CNT结构。添加柠檬酸会在层状g-C 3 N 4上引入无定形碳源-Ni和在800℃的相同温度下退火后,代替形成NiSA-N-CNT,获得了嵌入在平面石墨烯型载体中的Ni单原子NiSA-NG。密度泛函理论(DFT)的计算表明,引入无定形碳源可显着降低层状g-C 3 N 4 - Ni中间产物的结构波动或曲率,从而中断固-固轧制过程并导致形成Ni单原子的平面石墨烯型载体的制备。Ni原子负载量约为6 wt%的催化剂合成的NiSA-NG表现出更好的活性和稳定性,对CO 2还原反应(CO 2与NiSA-N-CNT相比,由于NiSA-NG中具有开放和暴露的Ni单原子活性位,Ni原子负载约为15 wt%。这项研究首次证明了在SAC合成中控制碳载体的微观结构的可行性。
更新日期:2020-04-29
中文翻译:

可控的一锅法合成高负载碳纳米管和石墨烯载体中嵌入的镍单原子
单原子催化剂(SAC)由于具有高催化效率和选择性的优势而备受关注。但是,SAC的可控和有效合成仍然是一个重大挑战。在本文中,我们报告了氮掺杂碳纳米管(NiSA-N-CNT)和氮掺杂石墨烯(NiSA-NG-G)上嵌入的镍单原子的受控单锅合成。NiSA-N-CNT的形成是由于在800°C的高温下热解过程中,从堆积和分层的掺杂Ni的g-C 3 N 4,g-C 3 N 4的固-固卷积机制引起的-Ni结构为管状CNT结构。添加柠檬酸会在层状g-C 3 N 4上引入无定形碳源-Ni和在800℃的相同温度下退火后,代替形成NiSA-N-CNT,获得了嵌入在平面石墨烯型载体中的Ni单原子NiSA-NG。密度泛函理论(DFT)的计算表明,引入无定形碳源可显着降低层状g-C 3 N 4 - Ni中间产物的结构波动或曲率,从而中断固-固轧制过程并导致形成Ni单原子的平面石墨烯型载体的制备。Ni原子负载量约为6 wt%的催化剂合成的NiSA-NG表现出更好的活性和稳定性,对CO 2还原反应(CO 2与NiSA-N-CNT相比,由于NiSA-NG中具有开放和暴露的Ni单原子活性位,Ni原子负载约为15 wt%。这项研究首次证明了在SAC合成中控制碳载体的微观结构的可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号