Journal of CO2 Utilization ( IF 7.7 ) Pub Date : 2020-04-09 , DOI: 10.1016/j.jcou.2020.101159 Aravind V. Rayer , Elliot Reid , Atish Kataria , Ignacio Luz , Samuel J. Thompson , Marty Lail , James Zhou , Mustapha Soukri
|
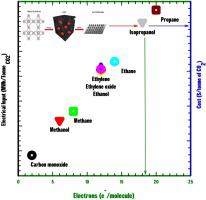
It is well known in sustainable energy research that metallic copper functions as an electrocatalyst for the reduction of CO2 to multicarbon products such as alcohols and hydrocarbons. However, it remains a great challenge to develop a cost-effective, selective, and stable catalyst/electrode material for this reaction. This work furthers previous studies concerning the potential of carbonized copper MOF-derived electrocatalysts as catalyst materials for the electrochemical reduction of CO2. Two commercial copper-decorated metal organic frameworks (MOFs), HKUST-1 and PCN-62, pyrolyzed at variable temperatures, 400−800 °C, were coated on both metallic nickel and copper supports as inks. The electrocatalysts’ potential to reduce CO2 was gauged using an electrochemical cell with both GC-TCD and GC-FID analyses. Many of the previously reported products of this reaction were formed, however, most notably, GC-FID analysis confirmed the formation of isopropanol, a product not previously reported to the best of our knowledge. It was observed that MOF-derived coatings can produce electrodes with both better current density and selectivity towards isopropanol compared to that of uncoated copper electrodes. Amongst all the carbon products observed, the best performing electrocatalyst demonstrated isopropanol faradaic efficiency (FE) of over 72 %. Preliminary techno-economic analysis was conducted to identify target cell operating voltage in order to make the proposed electrochemical CO2 reduction process economically feasible. The desired cell operating voltage is less than 1.9 V, preferably less than 1.6 V. Future work will focus on electrochemical cell design and electrocatalyst morphology to further improve isopropanol selectivity and minimize hydrogen FE.
中文翻译:

使用新型碳化铜金属有机骨架衍生电极将电化学二氧化碳还原为异丙醇
在可持续能源研究中众所周知,金属铜充当将CO 2还原为多碳产物(例如醇和烃)的电催化剂。然而,为该反应开发成本有效,选择性和稳定的催化剂/电极材料仍然是巨大的挑战。这项工作进一步开展了有关碳化铜MOF衍生电催化剂作为电化学还原CO 2催化剂材料的潜力的先前研究。将两种商业用铜装饰的金属有机框架HKUST-1和PCN-62(在400-800°C的可变温度下热解)涂在金属镍和铜载体上作为墨水。电催化剂降低CO 2的潜力使用带有GC-TCD和GC-FID分析的电化学池对样品进行测量。许多先前报道的该反应产物已经形成,但是,最值得注意的是,GC-FID分析证实了异丙醇的形成,据我们所知,该产物以前并未报道。观察到,与未涂覆的铜电极相比,MOF衍生的涂层可以生产具有更好的电流密度和对异丙醇选择性的电极。在观察到的所有碳产物中,性能最好的电催化剂显示出异丙醇法拉第效率(FE)超过72%。进行了初步的技术经济分析,以确定目标电池的工作电压,以制备拟议的电化学CO 2还原过程在经济上是可行的。所需的电池工作电压小于1.9 V,最好小于1.6V。未来的工作将集中在电化学电池设计和电催化剂形态上,以进一步提高异丙醇的选择性并使氢FE最小化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号