Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visualization of Ligand-Bound Ectodomain Assembly in the Full-Length Human IGF-1 Receptor by Cryo-EM Single-Particle Analysis.
Structure ( IF 4.4 ) Pub Date : 2020-04-09 , DOI: 10.1016/j.str.2020.03.007 Xi Zhang 1 , Daqi Yu 2 , Jingchuan Sun 3 , Yujie Wu 3 , Junyuan Gong 4 , Xuemei Li 5 , Li Liu 4 , Shan Liu 4 , Jianbo Liu 3 , Yulan Wu 4 , Dongyang Li 4 , Yinping Ma 6 , Xu Han 2 , Yanan Zhu 2 , Zhaolong Wu 2 , Yihua Wang 2 , Qi Ouyang 7 , Tao Wang 8
Structure ( IF 4.4 ) Pub Date : 2020-04-09 , DOI: 10.1016/j.str.2020.03.007 Xi Zhang 1 , Daqi Yu 2 , Jingchuan Sun 3 , Yujie Wu 3 , Junyuan Gong 4 , Xuemei Li 5 , Li Liu 4 , Shan Liu 4 , Jianbo Liu 3 , Yulan Wu 4 , Dongyang Li 4 , Yinping Ma 6 , Xu Han 2 , Yanan Zhu 2 , Zhaolong Wu 2 , Yihua Wang 2 , Qi Ouyang 7 , Tao Wang 8
Affiliation
|
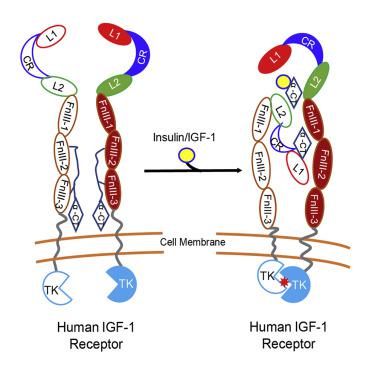
Tyrosine kinase receptor of insulin-like growth factor 1 receptor (IGF-1R) and insulin receptor (IR) bind to hormones, such as insulin, IGF-1, and IGF-2, and transduces the signals across the cell membrane. However, the complete structure of the receptor and the signal transduction mechanism remains unclear. Here, we report the cryo-EM structure of the ligand-bound ectodomain in the full-length human IGF-1R. We reconstructed the IGF-1R/insulin complex at 4.7 Å and the IGF-1R/IGF-1 complex at 7.7 Å. Our structures reveal that only one insulin or one IGF-1 molecule binds to and activates the full-length human IGF-1R receptor.
中文翻译:

通过低温-EM单颗粒分析可视化全长人IGF-1受体中配体结合的电子域结构。
胰岛素样生长因子1受体(IGF-1R)和胰岛素受体(IR)的酪氨酸激酶受体与诸如胰岛素,IGF-1和IGF-2的激素结合,并在细胞膜上传递信号。但是,受体的完整结构和信号转导机制仍不清楚。在这里,我们报道了全长人IGF-1R中配体结合的胞外域的cryo-EM结构。我们在4.7Å处重建了IGF-1R /胰岛素复合物,在7.7Å处重建了IGF-1R / IGF-1复合物。我们的结构揭示,只有一种胰岛素或一种IGF-1分子结合并激活全长人IGF-1R受体。
更新日期:2020-04-09
中文翻译:

通过低温-EM单颗粒分析可视化全长人IGF-1受体中配体结合的电子域结构。
胰岛素样生长因子1受体(IGF-1R)和胰岛素受体(IR)的酪氨酸激酶受体与诸如胰岛素,IGF-1和IGF-2的激素结合,并在细胞膜上传递信号。但是,受体的完整结构和信号转导机制仍不清楚。在这里,我们报道了全长人IGF-1R中配体结合的胞外域的cryo-EM结构。我们在4.7Å处重建了IGF-1R /胰岛素复合物,在7.7Å处重建了IGF-1R / IGF-1复合物。我们的结构揭示,只有一种胰岛素或一种IGF-1分子结合并激活全长人IGF-1R受体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号