当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Function and Aggregation in Structural Eye Lens Crystallins.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-09 , DOI: 10.1021/acs.accounts.0c00014 Kyle W Roskamp 1 , Carolyn N Paulson 2 , William D Brubaker 3 , Rachel W Martin 1, 4
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-09 , DOI: 10.1021/acs.accounts.0c00014 Kyle W Roskamp 1 , Carolyn N Paulson 2 , William D Brubaker 3 , Rachel W Martin 1, 4
Affiliation
|
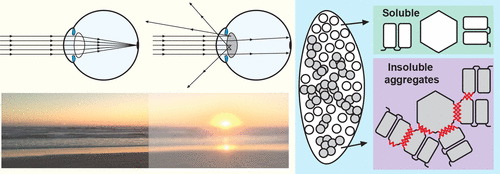
ConspectusCrystallins are transparent, refractive proteins that contribute to the focusing power of the vertebrate eye lens. These proteins are extremely soluble and resist aggregation for decades, even under crowded conditions. Crystallins have evolved to avoid strong interprotein interactions and have unusual hydration properties. Crystallin aggregation resulting from mutation, damage, or aging can lead to cataract, a disease state characterized by opacity of the lens.Different aggregation mechanisms can occur, following multiple pathways and leading to aggregates with varied morphologies. Studies of variant proteins found in individuals with childhood-onset cataract have provided insight into the molecular factors underlying crystallin stability and solubility. Modulation of exposed hydrophobic surface is critical, as is preventing specific intermolecular interactions that could provide nucleation sites for aggregation. Biophysical measurements and structural biology techniques are beginning to provide a detailed picture of how crystallins crowd into the lens, providing high refractivity while avoiding excessively tight binding that would lead to aggregation.Despite the central biological importance of refractivity, relatively few experimental measurements have been made for lens crystallins. Our work and that of others have shown that hydration is important to the high refractive index of crystallin proteins, as are interactions between pairs of aromatic residues and potentially other specific structural features.This Account describes our efforts to understand both the functional and disease states of vertebrate eye lens crystallins, particularly the γ-crystallins. We use a variety of biophysical techniques, notably NMR spectroscopy, to investigate crystallin stability and solubility. In the first section, we describe efforts to understand the relative stability and aggregation propensity of different γS-crystallin variants. The second section focuses on interactions of these proteins with the holdase chaperone αB-crystallin. The third, fourth, and fifth sections explore different modes of aggregation available to crystallin proteins, and the final section highlights the importance of refractive index and the sometimes conflicting demands of selection for refractivity and solubility.
中文翻译:

功能和聚集在结构性晶状体晶状体中。
晶体蛋白是透明的折射蛋白,有助于脊椎动物晶状体的聚焦能力。这些蛋白质极易溶解,即使在拥挤的条件下也能抵抗数十年的聚集。晶状体蛋白可以避免强烈的蛋白间相互作用,并具有异常的水合特性。由突变,损伤或衰老引起的晶状体蛋白聚集可导致白内障,即以晶状体不透明为特征的疾病状态,可通过多种途径发生不同的聚集机制,并导致形成形态各异的聚集体。对儿童期白内障患者中发现的变异蛋白的研究为深入了解晶状蛋白稳定性和溶解性的分子因素提供了见解。暴露的疏水表面的调节至关重要,如防止特定的分子间相互作用可能为聚集提供成核位点。生物物理测量和结构生物学技术开始提供有关晶状体蛋白如何挤入晶状体的详细图片,提供高折射率,同时避免过分紧密的结合会导致聚集。尽管折射率具有重要的生物学意义,但已经进行了相对较少的实验测量用于晶状体晶体。我们的工作和其他研究表明水合对结晶蛋白的高折射率很重要,成对的芳香残基与潜在的其他特定结构特征之间的相互作用也很重要。脊椎动物的晶状体晶体,特别是γ-晶状蛋白。我们使用各种生物物理技术(尤其是NMR光谱)来研究结晶蛋白的稳定性和溶解性。在第一部分中,我们描述了了解不同γS-晶状蛋白变异体的相对稳定性和聚集倾向的努力。第二部分重点介绍这些蛋白质与Holdase伴侣αB-crystallin的相互作用。第三,第四和第五部分探讨了可用于晶状体蛋白的不同聚集方式,最后一部分强调了折射率的重要性以及有时对折射率和溶解度的选择有冲突。我们描述了了解不同γS-晶状蛋白变异体的相对稳定性和聚集倾向的努力。第二部分着重于这些蛋白质与保持酶伴侣αB-晶状体蛋白的相互作用。第三,第四和第五部分探讨了可用于晶状体蛋白的不同聚集模式,最后一部分突出了折射率的重要性以及有时对折射率和溶解度的选择有冲突。我们描述了了解不同γS-晶状蛋白变异体的相对稳定性和聚集倾向的努力。第二部分重点研究这些蛋白质与Holdase伴侣αB-crystallin的相互作用。第三,第四和第五部分探讨了可用于晶状体蛋白的不同聚集模式,最后一部分突出了折射率的重要性以及有时对折射率和溶解度的选择有冲突。
更新日期:2020-04-23
中文翻译:

功能和聚集在结构性晶状体晶状体中。
晶体蛋白是透明的折射蛋白,有助于脊椎动物晶状体的聚焦能力。这些蛋白质极易溶解,即使在拥挤的条件下也能抵抗数十年的聚集。晶状体蛋白可以避免强烈的蛋白间相互作用,并具有异常的水合特性。由突变,损伤或衰老引起的晶状体蛋白聚集可导致白内障,即以晶状体不透明为特征的疾病状态,可通过多种途径发生不同的聚集机制,并导致形成形态各异的聚集体。对儿童期白内障患者中发现的变异蛋白的研究为深入了解晶状蛋白稳定性和溶解性的分子因素提供了见解。暴露的疏水表面的调节至关重要,如防止特定的分子间相互作用可能为聚集提供成核位点。生物物理测量和结构生物学技术开始提供有关晶状体蛋白如何挤入晶状体的详细图片,提供高折射率,同时避免过分紧密的结合会导致聚集。尽管折射率具有重要的生物学意义,但已经进行了相对较少的实验测量用于晶状体晶体。我们的工作和其他研究表明水合对结晶蛋白的高折射率很重要,成对的芳香残基与潜在的其他特定结构特征之间的相互作用也很重要。脊椎动物的晶状体晶体,特别是γ-晶状蛋白。我们使用各种生物物理技术(尤其是NMR光谱)来研究结晶蛋白的稳定性和溶解性。在第一部分中,我们描述了了解不同γS-晶状蛋白变异体的相对稳定性和聚集倾向的努力。第二部分重点介绍这些蛋白质与Holdase伴侣αB-crystallin的相互作用。第三,第四和第五部分探讨了可用于晶状体蛋白的不同聚集方式,最后一部分强调了折射率的重要性以及有时对折射率和溶解度的选择有冲突。我们描述了了解不同γS-晶状蛋白变异体的相对稳定性和聚集倾向的努力。第二部分着重于这些蛋白质与保持酶伴侣αB-晶状体蛋白的相互作用。第三,第四和第五部分探讨了可用于晶状体蛋白的不同聚集模式,最后一部分突出了折射率的重要性以及有时对折射率和溶解度的选择有冲突。我们描述了了解不同γS-晶状蛋白变异体的相对稳定性和聚集倾向的努力。第二部分重点研究这些蛋白质与Holdase伴侣αB-crystallin的相互作用。第三,第四和第五部分探讨了可用于晶状体蛋白的不同聚集模式,最后一部分突出了折射率的重要性以及有时对折射率和溶解度的选择有冲突。











































 京公网安备 11010802027423号
京公网安备 11010802027423号