当前位置:
X-MOL 学术
›
Food Sci. Nutr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acute and subchronic toxicity study of nonpolar extract of licorice roots in mice.
Food Science & Nutrition ( IF 3.5 ) Pub Date : 2020-04-08 , DOI: 10.1002/fsn3.1465 Hyun-Yong Kim 1 , Guanglei Zuo 1 , Soo Kyeong Lee 1, 2 , Soon Sung Lim 1, 2
Food Science & Nutrition ( IF 3.5 ) Pub Date : 2020-04-08 , DOI: 10.1002/fsn3.1465 Hyun-Yong Kim 1 , Guanglei Zuo 1 , Soo Kyeong Lee 1, 2 , Soon Sung Lim 1, 2
Affiliation
|
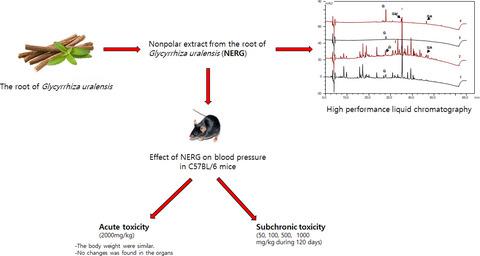
Licorice is used as a medicinal plant, and several studies have shown that licorice has beneficial effects. The objective of this study was to evaluate the safety of nonpolar licorice extract using toxicity experiments. Nonpolar extract from the root of Glycyrrhiza uralensis (NERG) was analyzed by high‐performance liquid chromatography (HPLC). Antioxidant ability was determined by method of TPC and DPPH. Blood pressure was monitored by using blood pressure meter. In the acute study, a single dose (2,000 mg/kg) was orally administered to mice. In the subchronic study, mice were treated with extract at doses (50, 100, 500, and 1,000 mg/kg) for 120 days. Significantly difference was not shown at blood pressure, hematological, and biochemical parameters, and histopathology on mice. The results suggested that at acute and subchronic toxicity, each levels of nonpolar licorice extract administration in experiments did not cause toxicity effects or death on mice.
中文翻译:

甘草根非极性提取物对小鼠的急性和亚慢性毒性研究。
甘草被用作药用植物,多项研究表明甘草具有有益的作用。本研究的目的是通过毒性实验评估非极性甘草提取物的安全性。通过高效液相色谱 (HPLC) 分析乌拉尔甘草根的非极性提取物 (NERG)。采用TPC和DPPH法测定抗氧化能力。使用血压计监测血压。在急性研究中,小鼠口服单剂量(2,000 mg/kg)。在亚慢性研究中,小鼠接受剂量(50、100、500 和 1,000 毫克/千克)的提取物治疗 120 天。小鼠的血压、血液学和生化参数以及组织病理学未显示出显着差异。结果表明,在急性和亚慢性毒性下,实验中给予各浓度的非极性甘草提取物均不会对小鼠产生毒性作用或死亡。
更新日期:2020-04-08
中文翻译:

甘草根非极性提取物对小鼠的急性和亚慢性毒性研究。
甘草被用作药用植物,多项研究表明甘草具有有益的作用。本研究的目的是通过毒性实验评估非极性甘草提取物的安全性。通过高效液相色谱 (HPLC) 分析乌拉尔甘草根的非极性提取物 (NERG)。采用TPC和DPPH法测定抗氧化能力。使用血压计监测血压。在急性研究中,小鼠口服单剂量(2,000 mg/kg)。在亚慢性研究中,小鼠接受剂量(50、100、500 和 1,000 毫克/千克)的提取物治疗 120 天。小鼠的血压、血液学和生化参数以及组织病理学未显示出显着差异。结果表明,在急性和亚慢性毒性下,实验中给予各浓度的非极性甘草提取物均不会对小鼠产生毒性作用或死亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号