当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Time-resolved infra-red spectroscopy reveals competitive water and dinitrogen coordination to a manganese(i) carbonyl complex.
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-04-07 , DOI: 10.1039/c9dt04878b Jonathan B Eastwood 1 , L Anders Hammarback , Matthew T McRobie , Ian P Clark , Michael Towrie , Ian J S Fairlamb , Jason M Lynam
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-04-07 , DOI: 10.1039/c9dt04878b Jonathan B Eastwood 1 , L Anders Hammarback , Matthew T McRobie , Ian P Clark , Michael Towrie , Ian J S Fairlamb , Jason M Lynam
Affiliation
|
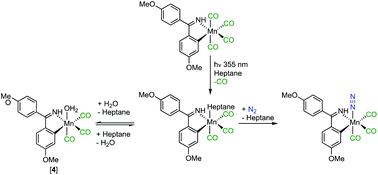
Time-resolved infra-red (TRIR) spectroscopy has been used to demonstrate that photolysis of [Mn(C^N)(CO)4] (C^N = bis-(4-methoxyphenyl)methanimine) in heptane solution results in ultra-fast CO dissociation and ultimate formation of a rare Mn-containing dinitrogen complex fac-[Mn(C^N)(CO)3(N2)] with a diagnostic stretching mode for a terminal-bound N[triple bond, length as m-dash]N ligand at 2249 cm-1. An isotopic shift to 2174 cm-1 was observed when the reaction was performed under 15N2 and the band was not present when the experiment was undertaken under an atmosphere of argon, reinforcing this assignment. An intermediate solvent complex fac-[Mn(C^N)(CO)3(heptane)] was identified which is formed in less than 2 ps, indicating that CO-release occurs on an ultra-fast timescale. The heptane ligand is labile and is readily displaced by both N2 and water to give fac-[Mn(C^N)(CO)3(N2)] and fac-[Mn(C^N)(CO)3(OH2)] respectively. The fac-[Mn(C^N)(CO)3(heptane)] framework showed a significant affinity for N2, as performing the reaction under air produced significant amounts of fac-[Mn(C^N)(CO)3(N2)]. Kinetic analysis reveals that the substitution of heptane by N2 (k = (1.028 ± 0.004) × 109 mol-1 dm3 s-1), and H2O is competitive on fast (<1 μs) time scales. The binding of water is reversible and, under an atmosphere of N2, some fac-[Mn(C^N)(CO)3(OH2)] converts to fac-[Mn(C^N)(CO)3(N2)].
中文翻译:

时间分辨的红外光谱揭示了锰(i)羰基配合物的竞争性水和二氮配位。
时间分辨红外(TRIR)光谱已被用于证明庚烷溶液中的[Mn(C ^ N)(CO)4](C ^ N =双-(4-甲氧基苯基)甲亚胺)的光解导致超-CO快速分解并最终形成稀有的含锰二氮配合物fac- [Mn(C ^ N)(CO)3(N2)],且具有诊断性的末端键合N [三键,长度为m的拉伸方式-在2249cm-1处的N配体。当反应在15N2下进行时,观察到同位素迁移至2174 cm-1,当在氩气气氛下进行实验时,该谱带不存在,从而加强了这种分配。鉴定了以小于2 ps的速度形成的中间溶剂复合物fac- [Mn(C ^ N)(CO)3(庚烷)],表明CO的释放在超快的时间尺度上发生。庚烷配体不稳定,容易被氮气和水置换,得到fac- [Mn(C ^ N)(CO)3(N2)]和fac- [Mn(C ^ N)(CO)3(OH2) ] 分别。fac- [Mn(C ^ N)(CO)3(庚烷)]骨架显示出对N2的显着亲和力,因为在空气中进行反应会产生大量的fac- [Mn(C ^ N)(CO)3( N2)]。动力学分析表明,庚烷被N2(k =(1.028±0.004)×109 mol-1 dm3 s-1)取代,H2O在快速(<1μs)的时间尺度上具有竞争力。水的结合是可逆的,在N2气氛下,某些fac- [Mn(C ^ N)(CO)3(OH2)]转化为fac- [Mn(C ^ N)(CO)3(N2) ]。在空气中进行反应时,生成大量的fac- [Mn(C ^ N)(CO)3(N2)]。动力学分析表明,庚烷被N2(k =(1.028±0.004)×109 mol-1 dm3 s-1)取代,H2O在快速(<1μs)的时间尺度上具有竞争力。水的结合是可逆的,在N2气氛下,某些fac- [Mn(C ^ N)(CO)3(OH2)]转化为fac- [Mn(C ^ N)(CO)3(N2) ]。在空气中进行反应时,生成大量的fac- [Mn(C ^ N)(CO)3(N2)]。动力学分析表明,庚烷被N2(k =(1.028±0.004)×109 mol-1 dm3 s-1)取代,H2O在快速(<1μs)的时间尺度上具有竞争力。水的结合是可逆的,在N2气氛下,某些fac- [Mn(C ^ N)(CO)3(OH2)]转化为fac- [Mn(C ^ N)(CO)3(N2) ]。
更新日期:2020-04-07
中文翻译:

时间分辨的红外光谱揭示了锰(i)羰基配合物的竞争性水和二氮配位。
时间分辨红外(TRIR)光谱已被用于证明庚烷溶液中的[Mn(C ^ N)(CO)4](C ^ N =双-(4-甲氧基苯基)甲亚胺)的光解导致超-CO快速分解并最终形成稀有的含锰二氮配合物fac- [Mn(C ^ N)(CO)3(N2)],且具有诊断性的末端键合N [三键,长度为m的拉伸方式-在2249cm-1处的N配体。当反应在15N2下进行时,观察到同位素迁移至2174 cm-1,当在氩气气氛下进行实验时,该谱带不存在,从而加强了这种分配。鉴定了以小于2 ps的速度形成的中间溶剂复合物fac- [Mn(C ^ N)(CO)3(庚烷)],表明CO的释放在超快的时间尺度上发生。庚烷配体不稳定,容易被氮气和水置换,得到fac- [Mn(C ^ N)(CO)3(N2)]和fac- [Mn(C ^ N)(CO)3(OH2) ] 分别。fac- [Mn(C ^ N)(CO)3(庚烷)]骨架显示出对N2的显着亲和力,因为在空气中进行反应会产生大量的fac- [Mn(C ^ N)(CO)3( N2)]。动力学分析表明,庚烷被N2(k =(1.028±0.004)×109 mol-1 dm3 s-1)取代,H2O在快速(<1μs)的时间尺度上具有竞争力。水的结合是可逆的,在N2气氛下,某些fac- [Mn(C ^ N)(CO)3(OH2)]转化为fac- [Mn(C ^ N)(CO)3(N2) ]。在空气中进行反应时,生成大量的fac- [Mn(C ^ N)(CO)3(N2)]。动力学分析表明,庚烷被N2(k =(1.028±0.004)×109 mol-1 dm3 s-1)取代,H2O在快速(<1μs)的时间尺度上具有竞争力。水的结合是可逆的,在N2气氛下,某些fac- [Mn(C ^ N)(CO)3(OH2)]转化为fac- [Mn(C ^ N)(CO)3(N2) ]。在空气中进行反应时,生成大量的fac- [Mn(C ^ N)(CO)3(N2)]。动力学分析表明,庚烷被N2(k =(1.028±0.004)×109 mol-1 dm3 s-1)取代,H2O在快速(<1μs)的时间尺度上具有竞争力。水的结合是可逆的,在N2气氛下,某些fac- [Mn(C ^ N)(CO)3(OH2)]转化为fac- [Mn(C ^ N)(CO)3(N2) ]。











































 京公网安备 11010802027423号
京公网安备 11010802027423号