Scientific Reports ( IF 4.6 ) Pub Date : 2020-04-08 , DOI: 10.1038/s41598-020-62961-5 Fen Wang 1 , Xia Yuan 2 , Jun Jia 3 , Xiaoxia Bi 4 , Zeqiang Zhou 5 , Qiming Zhou 6 , Xia Li 7 , Changguo Luo 8 , Minghui Deng 9 , Liangjie Yi 10 , Yong Li 11 , Jianxin Lu 12 , Wenzhi Su 13 , Hanbin Chen 14 , Yu Zhu 1, 15 , Shubin Wang 1, 15
|
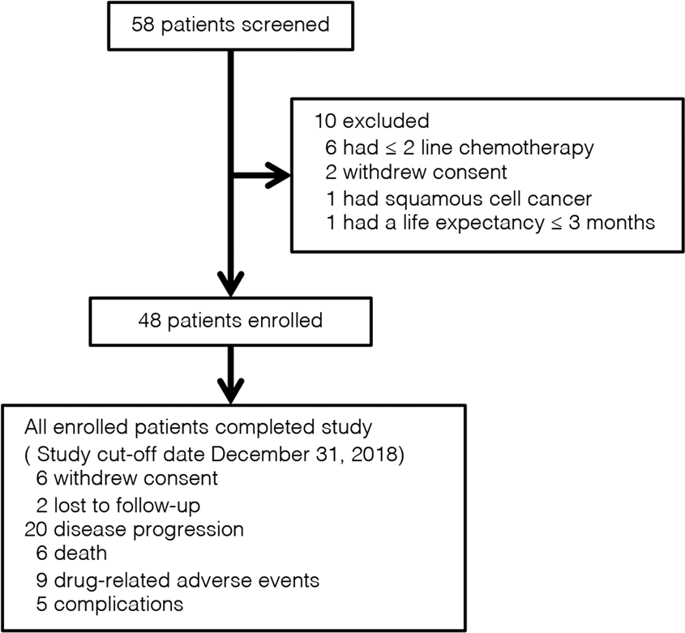
Angiogenesis inhibitors are of considerable interest for treating metastatic colorectal cancer (mCRC). This trial evaluated the efficacy and safety of apatinib in chemotherapy-refractory mCRC. Apatinib 500 mg was administered daily to patients who had progressed after two or more lines of standard fluorouracil-based chemotherapy. Primary endpoint was progression-free survival (PFS). Secondary endpoints were objective response rate (ORR), disease control rate (DCR), overall survival (OS), and toxicity. Overall, 48 patients were enrolled. ORR and DCR were 8.3% (4/48) and 68.8% (33/48), respectively. Median PFS and OS were 4.8 (95% confidence interval [CI], 3.653–5.887) and 9.1 months (95% CI, 5.155–13.045), respectively, and did not differ between subgroups stratified by previous anti-angiogenic therapies. The most prevalent grade 3–4 adverse events were hypertension (12.5%), hand-foot syndrome (HFS, 10.4%), thrombocytopenia (10.4%), and proteinuria (8.3%). Low baseline neutrophil/lymphocyte ratio (NLR, hazard ratios [HR], 0.619; P = 0.027), early carbohydrate antigen 19–9 (CA19–9) decrease (HR, 1.654; P = 0.016), and HFS (HR, 2.087; P = 0.007) were associated with improved PFS. In conclusion, apatinib monotherapy demonstrated encouraging efficacy with manageable toxicities in chemotherapy-refractory mCRC. Previous anti-angiogenic therapies did not influence outcomes. Baseline NLR, early CA19-9 decrease, and HFS could predict the efficacy of apatinib.
中文翻译:

阿帕替尼单药治疗难治性转移性结直肠癌:多中心,单臂,前瞻性研究。
血管生成抑制剂对于治疗转移性结直肠癌(mCRC)具有重要意义。该试验评估了阿帕替尼在难治性化疗中的疗效和安全性。每天向两行或更多行基于氟尿嘧啶的标准化学疗法后进展的患者每天给予500 mg阿帕替尼。主要终点是无进展生存期(PFS)。次要终点是客观缓解率(ORR),疾病控制率(DCR),总生存期(OS)和毒性。总共有48位患者入组。ORR和DCR分别为8.3%(4/48)和68.8%(33/48)。PFS和OS的中位数分别为4.8(95%置信区间[CI],3.653–5.887)和9.1个月(95%CI,5.155–13.045),并且在先前的抗血管生成治疗分层的亚组之间没有差异。最常见的3-4级不良事件是高血压(12.5%),手足综合征(HFS,10.4%),血小板减少症(10.4%)和蛋白尿(8.3%)。基线中性粒细胞/淋巴细胞比率低(NLR,危险比[HR],0.619; P = 0.027),早期碳水化合物抗原19–9(CA19–9)降低(HR,1.654; P = 0.016),HFS(HR,2.087) ; P = 0.007)与PFS改善有关。总之,阿帕替尼单药治疗在化疗难治性mCRC中显示出令人鼓舞的疗效和可控制的毒性。先前的抗血管生成疗法不会影响预后。基线NLR,早期CA19-9降低和HFS可以预测阿帕替尼的疗效。早期碳水化合物抗原19–9(CA19–9)降低(HR,1.654; P = 0.016)和HFS(HR,2.087; P = 0.007)与PFS改善相关。总之,阿帕替尼单药治疗在化疗难治性mCRC中显示出令人鼓舞的疗效和可控制的毒性。先前的抗血管生成疗法不会影响预后。基线NLR,早期CA19-9降低和HFS可以预测阿帕替尼的疗效。早期碳水化合物抗原19–9(CA19–9)降低(HR,1.654; P = 0.016)和HFS(HR,2.087; P = 0.007)与PFS改善相关。总之,阿帕替尼单药治疗在化疗难治性mCRC中显示出令人鼓舞的疗效和可控制的毒性。先前的抗血管生成疗法不会影响预后。基线NLR,早期CA19-9降低和HFS可以预测阿帕替尼的疗效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号