Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-08 , DOI: 10.1038/s41598-020-61427-y Shyamili Goutham 1 , Indu Kumari 2 , Dharma Pally 1 , Alvina Singh 1 , Sujasha Ghosh 1 , Yusuf Akhter 3 , Ramray Bhat 1
|
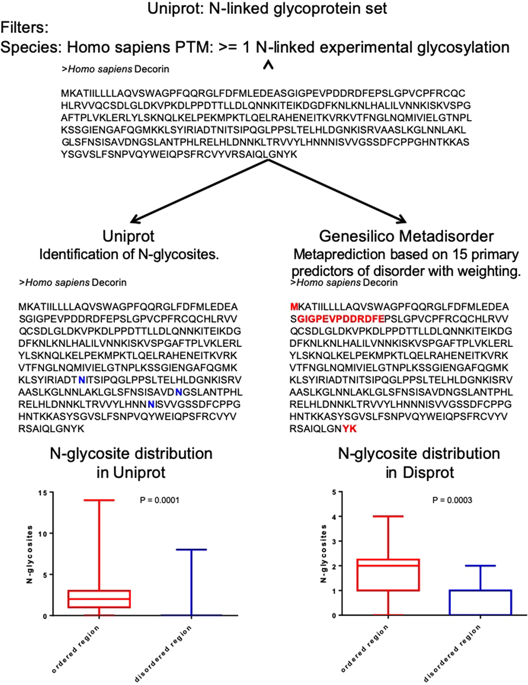
Several post-translational protein modifications lie predominantly within regions of disorder: the biased localization has been proposed to expand the binding versatility of disordered regions. However, investigating a representative dataset of 500 human N-glycoproteins, we observed the sites of N-linked glycosylations or N-glycosites, to be predominantly present in the regions of predicted order. When compared with disordered stretches, ordered regions were not found to be enriched for asparagines, serines and threonines, residues that constitute the sequon signature for conjugation of N-glycans. We then investigated the basis of mutual exclusivity between disorder and N-glycosites on the basis of amino acid distribution: when compared with control ordered residue stretches without any N-glycosites, residue neighborhoods surrounding N-glycosites showed a depletion of bulky, hydrophobic and disorder-promoting amino acids and an enrichment for flexible and accessible residues that are frequently found in coiled structures. When compared with control disordered residue stretches without any N-glycosites, N-glycosite neighborhoods were depleted of charged, polar, hydrophobic and flexible residues and enriched for aromatic, accessible and order-promoting residues with a tendency to be part of coiled and β structures. N-glycosite neighborhoods also showed greater phylogenetic conservation among amniotes, compared with control ordered regions, which in turn were more conserved than disordered control regions. Our results lead us to propose that unique primary structural compositions and differential propensities for evolvability allowed for the mutual spatial exclusion of N-glycosite neighborhoods and disordered stretches.
中文翻译:

N 连接聚糖的相互排斥区域和人类糖蛋白的紊乱。
几种翻译后蛋白质修饰主要位于无序区域内:已提出偏向定位以扩大无序区域的结合多功能性。然而,在研究 500 种人类 N-糖蛋白的代表性数据集时,我们观察到 N-连接糖基化或 N-糖位点的位点主要存在于预测顺序的区域中。与无序延伸相比,未发现有序区域富含天冬酰胺、丝氨酸和苏氨酸,这些残基构成了 N-聚糖缀合的序列序列特征。然后,我们根据氨基酸分布研究了无序和 N-糖位点之间相互排斥的基础:与没有任何 N-糖位点的对照有序残基延伸相比,N-糖位点周围的残基邻域显示出体积大、疏水性和无序性的缺失-促进氨基酸和富集在卷曲结构中常见的灵活且可接近的残基。与没有任何 N-糖位点的对照无序残基片段相比,N-糖位点附近的带电、极性、疏水性和柔性残基被耗尽,并且富含芳香族、可接近和促进有序的残基,并且倾向于成为卷曲和β结构的一部分。与控制有序区域相比,N-糖位点邻域在羊膜动物中也表现出更大的系统发育保守性,而控制有序区域又比无序控制区域更保守。我们的结果使我们提出,独特的一级结构组成和不同的进化倾向允许 N-糖位点邻域和无序延伸的空间相互排斥。











































 京公网安备 11010802027423号
京公网安备 11010802027423号