Nature ( IF 50.5 ) Pub Date : 2020-04-08 , DOI: 10.1038/s41586-020-2183-2 Daqian Xu , Zheng Wang , Yan Xia , Fei Shao , Weiya Xia , Yongkun Wei , Xinjian Li , Xu Qian , Jong-Ho Lee , Linyong Du , Yanhua Zheng , Guishuai Lv , Jia-shiun Leu , Hongyang Wang , Dongming Xing , Tingbo Liang , Mien-Chie Hung , Zhimin Lu
|
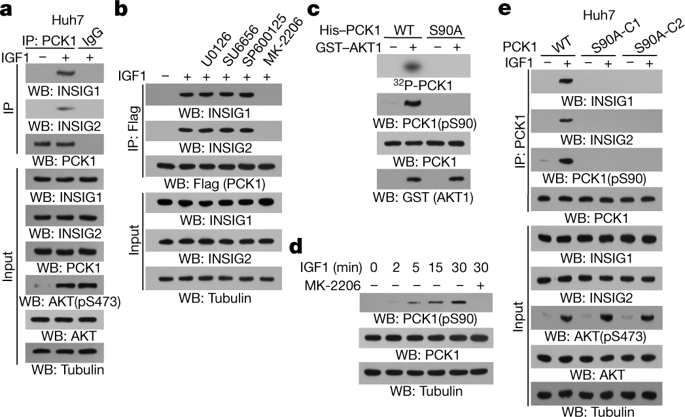
Cancer cells increase lipogenesis for their proliferation and the activation of sterol regulatory element-binding proteins (SREBPs) has a central role in this process. SREBPs are inhibited by a complex composed of INSIG proteins, SREBP cleavage-activating protein (SCAP) and sterols in the endoplasmic reticulum. Regulation of the interaction between INSIG proteins and SCAP by sterol levels is critical for the dissociation of the SCAP–SREBP complex from the endoplasmic reticulum and the activation of SREBPs1,2. However, whether this protein interaction is regulated by a mechanism other than the abundance of sterol—and in particular, whether oncogenic signalling has a role—is unclear. Here we show that activated AKT in human hepatocellular carcinoma (HCC) cells phosphorylates cytosolic phosphoenolpyruvate carboxykinase 1 (PCK1), the rate-limiting enzyme in gluconeogenesis, at Ser90. Phosphorylated PCK1 translocates to the endoplasmic reticulum, where it uses GTP as a phosphate donor to phosphorylate INSIG1 at Ser207 and INSIG2 at Ser151. This phosphorylation reduces the binding of sterols to INSIG1 and INSIG2 and disrupts the interaction between INSIG proteins and SCAP, leading to the translocation of the SCAP–SREBP complex to the Golgi apparatus, the activation of SREBP proteins (SREBP1 or SREBP2) and the transcription of downstream lipogenesis-related genes, proliferation of tumour cells, and tumorigenesis in mice. In addition, phosphorylation of PCK1 at Ser90, INSIG1 at Ser207 and INSIG2 at Ser151 is not only positively correlated with the nuclear accumulation of SREBP1 in samples from patients with HCC, but also associated with poor HCC prognosis. Our findings highlight the importance of the protein kinase activity of PCK1 in the activation of SREBPs, lipogenesis and the development of HCC.
中文翻译:

糖异生酶 PCK1 磷酸化 INSIG1/2 以促进脂肪生成
癌细胞会增加脂肪生成以促进其增殖,甾醇调节元件结合蛋白 (SREBP) 的激活在这一过程中起着核心作用。SREBP 被内质网中由 INSIG 蛋白、SREBP 裂解激活蛋白 (SCAP) 和甾醇组成的复合物抑制。通过甾醇水平调节 INSIG 蛋白和 SCAP 之间的相互作用对于 SCAP-SREBP 复合物与内质网的解离和 SREBP 的激活至关重要1,2. 然而,这种蛋白质相互作用是否受到甾醇丰度以外的机制的调节——特别是致癌信号是否起作用——尚不清楚。在这里,我们显示人肝细胞癌 (HCC) 细胞中活化的 AKT 在 Ser90 磷酸化胞质磷酸烯醇丙酮酸羧激酶 1 (PCK1),这是糖异生中的限速酶。磷酸化的 PCK1 易位到内质网,在那里它使用 GTP 作为磷酸供体来磷酸化 Ser207 位点的 INSIG1 和 Ser151 位点的 INSIG2。这种磷酸化降低了甾醇与 INSIG1 和 INSIG2 的结合,并破坏了 INSIG 蛋白和 SCAP 之间的相互作用,导致 SCAP-SREBP 复合物易位到高尔基体,SREBP 蛋白(SREBP1 或 SREBP2)的激活和下游脂肪生成相关基因的转录、肿瘤细胞的增殖和小鼠的肿瘤发生。此外,PCK1 在 Ser90、INSIG1 在 Ser207 和 INSIG2 在 Ser151 的磷酸化不仅与 HCC 患者样本中 SREBP1 的核积累呈正相关,而且与 HCC 预后不良有关。我们的研究结果强调了 PCK1 的蛋白激酶活性在激活 SREBP、脂肪生成和 HCC 发展中的重要性。但也与 HCC 预后不良有关。我们的研究结果强调了 PCK1 的蛋白激酶活性在激活 SREBP、脂肪生成和 HCC 发展中的重要性。但也与 HCC 预后不良有关。我们的研究结果强调了 PCK1 的蛋白激酶活性在激活 SREBP、脂肪生成和 HCC 发展中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号