Nature ( IF 50.5 ) Pub Date : 2020-04-08 , DOI: 10.1038/s41586-020-2174-3 Catherine R Lammert 1, 2 , Elizabeth L Frost 1 , Calli E Bellinger 1 , Ashley C Bolte 1, 3, 4 , Celia A McKee 1 , Mariah E Hurt 1 , Matt J Paysour 1 , Hannah E Ennerfelt 1, 2 , John R Lukens 1, 2
|
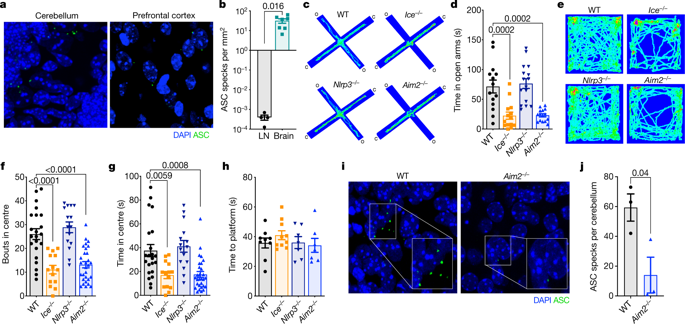
Neurodevelopment is characterized by rapid rates of neural cell proliferation and differentiation followed by massive cell death in which more than half of all recently generated brain cells are pruned back. Large amounts of DNA damage, cellular debris, and by-products of cellular stress are generated during these neurodevelopmental events, all of which can potentially activate immune signalling. How the immune response to this collateral damage influences brain maturation and function remains unknown. Here we show that the AIM2 inflammasome contributes to normal brain development and that disruption of this immune sensor of genotoxic stress leads to behavioural abnormalities. During infection, activation of the AIM2 inflammasome in response to double-stranded DNA damage triggers the production of cytokines as well as a gasdermin-D-mediated form of cell death known as pyroptosis1,2,3,4. We observe pronounced AIM2 inflammasome activation in neurodevelopment and find that defects in this sensor of DNA damage result in anxiety-related behaviours in mice. Furthermore, we show that the AIM2 inflammasome contributes to central nervous system (CNS) homeostasis specifically through its regulation of gasdermin-D, and not via its involvement in the production of the cytokines IL-1 and/or IL-18. Consistent with a role for this sensor of genomic stress in the purging of genetically compromised CNS cells, we find that defective AIM2 inflammasome signalling results in decreased neural cell death both in response to DNA damage-inducing agents and during neurodevelopment. Moreover, mutations in AIM2 lead to excessive accumulation of DNA damage in neurons as well as an increase in the number of neurons that incorporate into the adult brain. Our findings identify the inflammasome as a crucial player in establishing a properly formed CNS through its role in the removal of genetically compromised cells.
中文翻译:

AIM2 炎症小体对 DNA 损伤的监测影响神经发育
神经发育的特点是神经细胞增殖和分化速度很快,随后出现大量细胞死亡,其中一半以上最近生成的脑细胞被修剪掉。在这些神经发育事件中会产生大量 DNA 损伤、细胞碎片和细胞应激副产物,所有这些都可能激活免疫信号传导。对这种附带损伤的免疫反应如何影响大脑成熟和功能仍然未知。在这里,我们表明 AIM2 炎症小体有助于正常的大脑发育,而这种基因毒性应激免疫传感器的破坏会导致行为异常。在感染过程中,AIM2 炎症小体响应双链 DNA 损伤而激活,触发细胞因子的产生以及gasdermin-D 介导的细胞死亡形式(称为细胞焦亡) 1,2,3,4 。我们观察到神经发育过程中 AIM2 炎症小体的显着激活,并发现这种 DNA 损伤传感器的缺陷会导致小鼠出现焦虑相关行为。此外,我们发现 AIM2 炎性体具体通过其对 Gasdermin-D 的调节来促进中枢神经系统(CNS)稳态,而不是通过其参与细胞因子 IL-1 和/或 IL-18 的产生。与这种基因组应激传感器在清除基因受损的中枢神经系统细胞中的作用一致,我们发现有缺陷的 AIM2 炎性体信号传导导致神经细胞死亡减少,无论是对 DNA 损伤诱导剂的反应还是在神经发育过程中。此外,AIM2 的突变会导致神经元中 DNA 损伤过度积累,并导致融入成人大脑的神经元数量增加。 我们的研究结果表明,炎症小体通过其在去除基因受损细胞中的作用,在建立正确形成的中枢神经系统中发挥着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号