当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-catalyzed reductive carbonylation of aryl iodides to arylaldehydes with syngas.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-04-08 , DOI: 10.3762/bjoc.16.61 Zhenghui Liu 1 , Peng Wang 2, 3 , Zhenzhong Yan 1 , Suqing Chen 1 , Dongkun Yu 4 , Xinhui Zhao 4 , Tiancheng Mu 4
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-04-08 , DOI: 10.3762/bjoc.16.61 Zhenghui Liu 1 , Peng Wang 2, 3 , Zhenzhong Yan 1 , Suqing Chen 1 , Dongkun Yu 4 , Xinhui Zhao 4 , Tiancheng Mu 4
Affiliation
|
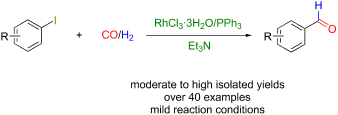
The reductive carbonylation of aryl iodides to aryl aldehydes possesses broad application prospects. We present an efficient and facile Rh-based catalytic system composed of the commercially available Rh salt RhCl3·3H2O, PPh3 as phosphine ligand, and Et3N as the base, for the synthesis of arylaldehydes via the reductive carbonylation of aryl iodides with CO and H2 under relatively mild conditions with a broad substrate range affording the products in good to excellent yields. Systematic investigations were carried out to study the experimental parameters. We explored the optimal ratio of Rh salt and PPh3 ligand, substrate scope, carbonyl source and hydrogen source, and the reaction mechanism. Particularly, a scaled-up experiment indicated that the catalytic method could find valuable applications in industrial productions. The low gas pressure, cheap ligand and low metal dosage could significantly improve the practicability in both chemical researches and industrial applications.
中文翻译:

铑催化的合成气将芳基碘化物还原羰基化为芳基醛。
芳基碘化物还原羰基化制备芳基醛具有广阔的应用前景。我们提出了一种高效、简便的Rh基催化体系,由市售的Rh盐RhCl3·3H2O、PPh3作为膦配体、Et3N作为碱组成,用于在CO和H2下通过芳基碘化物的还原羰基化合成芳基醛。相对温和的条件和广泛的底物范围使产品具有良好到优异的产率。对实验参数进行了系统研究。我们探讨了Rh盐与PPh3配体的最佳配比、底物范围、羰基源和氢源以及反应机理。特别是,放大实验表明催化方法可以在工业生产中找到有价值的应用。低气压、廉价配体和低金属用量可以显着提高化学研究和工业应用的实用性。
更新日期:2020-04-08
中文翻译:

铑催化的合成气将芳基碘化物还原羰基化为芳基醛。
芳基碘化物还原羰基化制备芳基醛具有广阔的应用前景。我们提出了一种高效、简便的Rh基催化体系,由市售的Rh盐RhCl3·3H2O、PPh3作为膦配体、Et3N作为碱组成,用于在CO和H2下通过芳基碘化物的还原羰基化合成芳基醛。相对温和的条件和广泛的底物范围使产品具有良好到优异的产率。对实验参数进行了系统研究。我们探讨了Rh盐与PPh3配体的最佳配比、底物范围、羰基源和氢源以及反应机理。特别是,放大实验表明催化方法可以在工业生产中找到有价值的应用。低气压、廉价配体和低金属用量可以显着提高化学研究和工业应用的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号