当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arsenic release from arsenopyrite oxidative dissolution in the presence of citrate under UV irradiation.
Science of the Total Environment ( IF 9.8 ) Pub Date : 2020-04-08 , DOI: 10.1016/j.scitotenv.2020.138429 Jun Hong 1 , Lihu Liu 1 , Wenfeng Tan 1 , Guohong Qiu 1
Science of the Total Environment ( IF 9.8 ) Pub Date : 2020-04-08 , DOI: 10.1016/j.scitotenv.2020.138429 Jun Hong 1 , Lihu Liu 1 , Wenfeng Tan 1 , Guohong Qiu 1
Affiliation
|
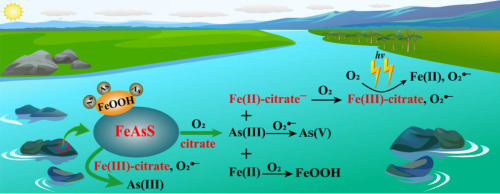
Arsenopyrite oxidative dissolution is one of the most important sources of arsenic (As) pollution in the soils and waters around sulfide mining areas. Sunlight and low-molecular-weight organic acids in the environment affect the redox behavior of sulfide minerals. In this work, the As release from arsenopyrite was studied in the presence of citrate under UV irradiation, and the effects of dissolved oxygen and citrate concentrations and pH on As release rate were also investigated. The results indicated that As release from the oxidative dissolution of arsenopyrite is affected by the complexation between citrate and dissolved iron ions. Under dark conditions in air atmosphere, dissolved oxygen, Fe(III)-citrate and the active intermediate product O2- facilitated the release of As at pH 7.0, and the As release rate increased first and then decreased with increasing pH from 5.0 to 9.0. Under UV irradiation in air atmosphere at pH 7.0, the reactive oxygen species (ROS) including O2- and OH generated by Fe(III)-citrate through the photo-Fenton reaction accelerated the As release and oxidation. However, Fe(III)-citrate photolysis led to the rapid flocculation and precipitation of dissolved iron ions, inhibiting the further oxidation of arsenopyrite. With increasing pH from 5.0 to 9.0, the As release rate gradually decreased under UV irradiation. Increases in the concentrations of citrate and dissolved oxygen promoted the formation of Fe(III)-citrate and ROS in the reaction system under both UV irradiation and dark conditions. The present work expands our understanding of the geochemical behavior of As in near-neutral pH environment.
中文翻译:

在柠檬酸盐存在下,紫外线照射下砷从毒砂的氧化溶解中释放出来的砷。
毒砂的氧化溶解是硫化矿开采区周围土壤和水体中砷(As)污染的最重要来源之一。环境中的阳光和低分子量有机酸会影响硫化物矿物的氧化还原行为。在这项工作中,研究了在柠檬酸盐存在下在紫外线照射下砷黄铁矿中砷的释放,并研究了溶解氧,柠檬酸盐浓度和pH对砷释放速率的影响。结果表明,砷从黄铁矿的氧化溶解中释放出来的砷受柠檬酸根与溶解的铁离子之间的络合作用影响。在空气气氛中的黑暗条件下,溶解的氧,柠檬酸三价铁和活性中间产物O2-促进了pH值为7.0时As的释放,随着pH值从5.0增加到9.0,As释放速率先增加然后降低。在pH值为7.0的大气中,在紫外线下,柠檬酸Fe(III)通过光芬顿反应生成的包括O2-和OH在内的活性氧(ROS)促进了As的释放和氧化。但是,柠檬酸Fe(III)的光解导致溶解的铁离子的快速絮凝和沉淀,从而抑制了毒砂的进一步氧化。随着pH从5.0增加到9.0,在紫外线照射下,As释放速率逐渐降低。柠檬酸盐和溶解氧浓度的增加促进了在紫外线辐射和黑暗条件下反应体系中柠檬酸三价铁和ROS的形成。本工作扩大了我们对砷在近中性pH环境中的地球化学行为的理解。
更新日期:2020-04-08
中文翻译:

在柠檬酸盐存在下,紫外线照射下砷从毒砂的氧化溶解中释放出来的砷。
毒砂的氧化溶解是硫化矿开采区周围土壤和水体中砷(As)污染的最重要来源之一。环境中的阳光和低分子量有机酸会影响硫化物矿物的氧化还原行为。在这项工作中,研究了在柠檬酸盐存在下在紫外线照射下砷黄铁矿中砷的释放,并研究了溶解氧,柠檬酸盐浓度和pH对砷释放速率的影响。结果表明,砷从黄铁矿的氧化溶解中释放出来的砷受柠檬酸根与溶解的铁离子之间的络合作用影响。在空气气氛中的黑暗条件下,溶解的氧,柠檬酸三价铁和活性中间产物O2-促进了pH值为7.0时As的释放,随着pH值从5.0增加到9.0,As释放速率先增加然后降低。在pH值为7.0的大气中,在紫外线下,柠檬酸Fe(III)通过光芬顿反应生成的包括O2-和OH在内的活性氧(ROS)促进了As的释放和氧化。但是,柠檬酸Fe(III)的光解导致溶解的铁离子的快速絮凝和沉淀,从而抑制了毒砂的进一步氧化。随着pH从5.0增加到9.0,在紫外线照射下,As释放速率逐渐降低。柠檬酸盐和溶解氧浓度的增加促进了在紫外线辐射和黑暗条件下反应体系中柠檬酸三价铁和ROS的形成。本工作扩大了我们对砷在近中性pH环境中的地球化学行为的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号