当前位置:
X-MOL 学术
›
Microbiologyopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodobacter capsulatus AnfA is essential for production of Fe-nitrogenase proteins but dispensable for cofactor biosynthesis and electron supply.
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-03-23 , DOI: 10.1002/mbo3.1033 Lisa Demtröder 1 , Yvonne Pfänder 1 , Bernd Masepohl 1
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-03-23 , DOI: 10.1002/mbo3.1033 Lisa Demtröder 1 , Yvonne Pfänder 1 , Bernd Masepohl 1
Affiliation
|
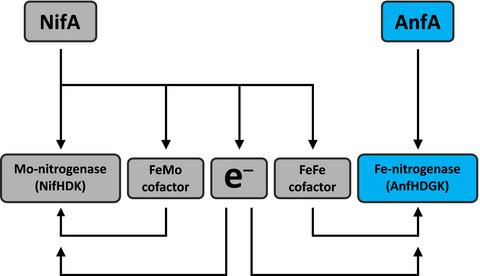
The photosynthetic α‐proteobacterium Rhodobacter capsulatus reduces and thereby fixes atmospheric dinitrogen (N2) by a molybdenum (Mo)‐nitrogenase and an iron‐only (Fe)‐nitrogenase. Differential expression of the structural genes of Mo‐nitrogenase (nifHDK) and Fe‐nitrogenase (anfHDGK) is strictly controlled and activated by NifA and AnfA, respectively. In contrast to NifA‐binding sites, AnfA‐binding sites are poorly defined. Here, we identified two highly similar AnfA‐binding sites in the R. capsulatus anfH promoter by studying the effects of promoter mutations on in vivo anfH expression and in vitro promoter binding by AnfA. Comparison of the experimentally determined R. capsulatus AnfA‐binding sites and presumed AnfA‐binding sites from other α‐proteobacteria revealed a consensus sequence of dyad symmetry, TAC–N6–GTA, suggesting that AnfA proteins bind their target promoters as dimers. Chromosomal replacement of the anfH promoter by the nifH promoter restored anfHDGK expression and Fe‐nitrogenase activity in an R. capsulatus strain lacking AnfA suggesting that AnfA is required for AnfHDGK production, but dispensable for biosynthesis of the iron‐only cofactor and electron delivery to Fe‐nitrogenase, pathways activated by NifA. These observations strengthen our model, in which the Fe‐nitrogenase system in R. capsulatus is largely integrated into the Mo‐nitrogenase system.
中文翻译:

荚膜红细菌AnfA对产生Fe-氮酶蛋白必不可少,但对于辅助因子的生物合成和电子供应而言却是必不可少的。
光合作用的α-变形杆菌Rhodobacter荚膜菌减少,从而通过钼(Mo)-硝化酶和仅铁(Fe)-硝化酶固定大气中的二氮(N 2)。Ni - A和AnfA分别严格控制和激活Mo-固氮酶(nifHDK)和Fe-固氮酶(anfHDGK)的结构基因的差异表达。与NifA结合位点相反,AnfA结合位点定义不清。在这里,我们通过研究启动子突变对体内anfH表达和AnfA体外启动子结合的影响,在荚膜红球菌anfH启动子中鉴定出两个高度相似的AnfA结合位点。实验确定的比较R.荚膜ANFA结合位点和来自其它α-变形菌推测ANFA结合位点揭示二重对称,TAC-N的共有序列6 -GTA,这表明ANFA蛋白结合其靶启动子二聚体。染色体更换的股骨头缺血性坏死受该启动子nifH启动子恢复anfHDGK在表达和Fe-固氮酶活R.荚膜缺乏ANFA表明ANFA需要AnfHDGK生产菌株,但可有可无的铁仅辅因子的生物合成和电子输送到铁氮酶,由NifA激活的途径。这些观察结果加强了我们的模型,在该模型中,荚膜红球菌中的铁氮酶系统 已基本整合到Mo-硝化酶系统中。
更新日期:2020-03-23
中文翻译:

荚膜红细菌AnfA对产生Fe-氮酶蛋白必不可少,但对于辅助因子的生物合成和电子供应而言却是必不可少的。
光合作用的α-变形杆菌Rhodobacter荚膜菌减少,从而通过钼(Mo)-硝化酶和仅铁(Fe)-硝化酶固定大气中的二氮(N 2)。Ni - A和AnfA分别严格控制和激活Mo-固氮酶(nifHDK)和Fe-固氮酶(anfHDGK)的结构基因的差异表达。与NifA结合位点相反,AnfA结合位点定义不清。在这里,我们通过研究启动子突变对体内anfH表达和AnfA体外启动子结合的影响,在荚膜红球菌anfH启动子中鉴定出两个高度相似的AnfA结合位点。实验确定的比较R.荚膜ANFA结合位点和来自其它α-变形菌推测ANFA结合位点揭示二重对称,TAC-N的共有序列6 -GTA,这表明ANFA蛋白结合其靶启动子二聚体。染色体更换的股骨头缺血性坏死受该启动子nifH启动子恢复anfHDGK在表达和Fe-固氮酶活R.荚膜缺乏ANFA表明ANFA需要AnfHDGK生产菌株,但可有可无的铁仅辅因子的生物合成和电子输送到铁氮酶,由NifA激活的途径。这些观察结果加强了我们的模型,在该模型中,荚膜红球菌中的铁氮酶系统 已基本整合到Mo-硝化酶系统中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号