当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubilities of 2,6‐Diamino‐3,5‐dinitropyrazine‐1‐oxide in the Binary Mixtures of DMSO+H2O, DMF+H2O and NMP+H2O in the Temperature Range from 293.15 to 323.15 K under the Atmospheric Pressure
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2019-11-27 , DOI: 10.1002/prep.201900155 Yuqiao Wang 1 , Shaohua Jin 1 , Tujuan Li 1 , Guanchao Lan 1 , Xiaopeng Zhang 1 , Zhengzheng Zhang 2 , Chang Zhou 3 , Yu Chen 1
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2019-11-27 , DOI: 10.1002/prep.201900155 Yuqiao Wang 1 , Shaohua Jin 1 , Tujuan Li 1 , Guanchao Lan 1 , Xiaopeng Zhang 1 , Zhengzheng Zhang 2 , Chang Zhou 3 , Yu Chen 1
Affiliation
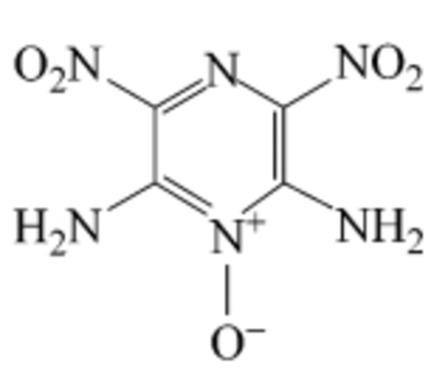
|
Recrystallization is usually required for the application of 2,6‐Diamino‐3,5‐dinitropyrazine‐1‐oxide (LLM‐105) as an energy material due to its poor crystal morphology. The study of its solubilities in binary solvent mixtures can provide primary support for optimizing the recrystallization and spheroidization conditions of LLM‐105. In the current work, the solubilities of LLM‐105 in the binary mixtures of dimethyl sulfoxide (DMSO)+H2O, N,N‐dimethyl‐formamide (DMF)+H2O and 1‐methyl‐2‐pyrrolidone (NMP) +H2O were measured by the gravimetric method in the temperature range from 293.15 K to 323.15 K under the atmospheric pressure. The results suggest that the solubility of LLM‐105 increases with the increase of temperature and the concentration of the solvent. The experimental data were then fitted with the modified Apelbalt equation, (CNIBS)/Redlich‐Kister equation and Jouyban‐Acree equation. All models fit the experimental data well, indicating that they can be used to predict the solubilities of LLM‐105 in the binary solvent mixtures. The thermodynamic parameters of the dissolution including enthalpy, standard entropy and Gibbs energy were calculated from the experimental data. It was found that the dissolutions of LLM‐105 in all test solvents were endothermic.
中文翻译:

在大气压下温度为293.15至323.15 K的DMSO + H2O,DMF + H2O和NMP + H2O的二元混合物中2,6-二氨基-3,5-二硝基吡嗪-1-氧化物的溶解度
由于2,6-二氨基-3,5-二硝基吡嗪-1-氧化物(LLM-105)由于其晶体形态较差,通常需要重结晶。研究其在二元溶剂混合物中的溶解度可为优化LLM-105的重结晶和球化条件提供主要支持。在当前工作中,LLM-105在二甲基亚砜(DMSO)+ H 2 O,N,N-二甲基甲酰胺(DMF)+ H 2 O和1-甲基-2-吡咯烷酮(NMP )的二元混合物中的溶解度)+ H 2在大气压下,在293.15 K至323.15 K的温度范围内通过重量分析法测量O。结果表明,LLM-105的溶解度随温度和溶剂浓度的增加而增加。然后,将实验数据与修改后的Apelbalt方程,(CNIBS)/ Redlich-Kister方程和Jouyban-Acree方程进行拟合。所有模型都很好地拟合了实验数据,表明它们可用于预测LLM-105在二元溶剂混合物中的溶解度。从实验数据计算出溶解的热力学参数,包括焓,标准熵和吉布斯能。结果发现,LLM-105在所有测试溶剂中的溶解都是吸热的。
更新日期:2020-03-22
中文翻译:

在大气压下温度为293.15至323.15 K的DMSO + H2O,DMF + H2O和NMP + H2O的二元混合物中2,6-二氨基-3,5-二硝基吡嗪-1-氧化物的溶解度
由于2,6-二氨基-3,5-二硝基吡嗪-1-氧化物(LLM-105)由于其晶体形态较差,通常需要重结晶。研究其在二元溶剂混合物中的溶解度可为优化LLM-105的重结晶和球化条件提供主要支持。在当前工作中,LLM-105在二甲基亚砜(DMSO)+ H 2 O,N,N-二甲基甲酰胺(DMF)+ H 2 O和1-甲基-2-吡咯烷酮(NMP )的二元混合物中的溶解度)+ H 2在大气压下,在293.15 K至323.15 K的温度范围内通过重量分析法测量O。结果表明,LLM-105的溶解度随温度和溶剂浓度的增加而增加。然后,将实验数据与修改后的Apelbalt方程,(CNIBS)/ Redlich-Kister方程和Jouyban-Acree方程进行拟合。所有模型都很好地拟合了实验数据,表明它们可用于预测LLM-105在二元溶剂混合物中的溶解度。从实验数据计算出溶解的热力学参数,包括焓,标准熵和吉布斯能。结果发现,LLM-105在所有测试溶剂中的溶解都是吸热的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号