当前位置:
X-MOL 学术
›
J. Rare Earths
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of cerium oxide prepared under different hydrothermal times on electrocatalytic performance of Pt - based anode catalysts
Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jre.2019.05.010 Ruihua Guo , Jie Wang , Shengli An , Jieyu Zhang , Guozhi Zhou , Lele Guo
Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jre.2019.05.010 Ruihua Guo , Jie Wang , Shengli An , Jieyu Zhang , Guozhi Zhou , Lele Guo
|
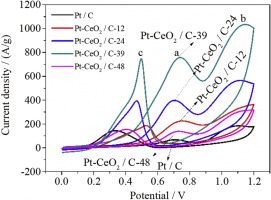
Abstract In this paper, CeO2 with a pore size of 2–4 nm was synthesized by hydrothermal method. The CeO2 modified graphene-supported Pt catalyst was prepared by the microwave-assisted ethylene glycol reduction chloroplatinic acid method, and the effect of the addition of CeO2 prepared by different hydrothermal reaction time on the catalytic performance of Pt-based catalysts was investigated. The microstructures of CeO2 and catalysts were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), specific surface area and pore size analyzer (BET), scanning electron microscopy (SEM) and electron spectroscopy (EDAX), transmission electron microscopy (TEM), and the catalysts electrochemical performance was tested by electrochemical workstation. The results show that the catalytic performance of the four catalysts with CeO2 is better than that of the catalyst without CeO2. Adding CeO2 with a specific surface area of 120.15 m2/g prepared by hydrothermal reaction time of 39 h to Pt/C synthesis catalyst, its electrocatalytic performance, stability and resistance to poisoning are the best. The electrochemical active surface area is 102.83 m2/g, the peak current density of ethanol oxidation is 757.17 A/g and steady-state current density of 1100 s is 108.17 A/g which shows the lowest activation energy for ethanol oxidation reaction. When the cyclic voltammogram is scanned for 500 cycles, the oxidation peak current density retention rate is 87.74%.
中文翻译:

不同水热时间下制备的氧化铈对铂基阳极催化剂电催化性能的影响
摘要 本文采用水热法合成了孔径为2~4 nm的CeO2。采用微波辅助乙二醇还原氯铂酸法制备了CeO2改性石墨烯负载Pt催化剂,研究了不同水热反应时间制备的CeO2添加量对Pt基催化剂催化性能的影响。通过X射线衍射(XRD)、X射线光电子能谱(XPS)、比表面积和孔径分析仪(BET)、扫描电子显微镜(SEM)和电子能谱(EDAX)对CeO2和催化剂的微观结构进行表征,透射电子显微镜(TEM),电化学工作站测试催化剂电化学性能。结果表明,含CeO2 的四种催化剂的催化性能均优于不含CeO2 的催化剂。将水热反应时间为39 h制备的比表面积为120.15 m2/g的CeO2加入Pt/C合成催化剂中,其电催化性能、稳定性和抗中毒能力均最佳。电化学活性表面积为102.83 m2/g,乙醇氧化的峰值电流密度为757.17 A/g,1100 s的稳态电流密度为108.17 A/g,表明乙醇氧化反应的活化能最低。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。15 m2/g 水热反应时间 39 h 制备的 Pt/C 合成催化剂,其电催化性能、稳定性和抗中毒性能均最佳。电化学活性表面积为102.83 m2/g,乙醇氧化的峰值电流密度为757.17 A/g,1100 s的稳态电流密度为108.17 A/g,表明乙醇氧化反应的活化能最低。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。15 m2/g 水热反应时间 39 h 制备的 Pt/C 合成催化剂,其电催化性能、稳定性和抗中毒性能均最佳。电化学活性表面积为102.83 m2/g,乙醇氧化的峰值电流密度为757.17 A/g,1100 s的稳态电流密度为108.17 A/g,表明乙醇氧化反应的活化能最低。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。17 A/g 显示了乙醇氧化反应的最低活化能。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。17 A/g 显示了乙醇氧化反应的最低活化能。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。
更新日期:2020-04-01
中文翻译:

不同水热时间下制备的氧化铈对铂基阳极催化剂电催化性能的影响
摘要 本文采用水热法合成了孔径为2~4 nm的CeO2。采用微波辅助乙二醇还原氯铂酸法制备了CeO2改性石墨烯负载Pt催化剂,研究了不同水热反应时间制备的CeO2添加量对Pt基催化剂催化性能的影响。通过X射线衍射(XRD)、X射线光电子能谱(XPS)、比表面积和孔径分析仪(BET)、扫描电子显微镜(SEM)和电子能谱(EDAX)对CeO2和催化剂的微观结构进行表征,透射电子显微镜(TEM),电化学工作站测试催化剂电化学性能。结果表明,含CeO2 的四种催化剂的催化性能均优于不含CeO2 的催化剂。将水热反应时间为39 h制备的比表面积为120.15 m2/g的CeO2加入Pt/C合成催化剂中,其电催化性能、稳定性和抗中毒能力均最佳。电化学活性表面积为102.83 m2/g,乙醇氧化的峰值电流密度为757.17 A/g,1100 s的稳态电流密度为108.17 A/g,表明乙醇氧化反应的活化能最低。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。15 m2/g 水热反应时间 39 h 制备的 Pt/C 合成催化剂,其电催化性能、稳定性和抗中毒性能均最佳。电化学活性表面积为102.83 m2/g,乙醇氧化的峰值电流密度为757.17 A/g,1100 s的稳态电流密度为108.17 A/g,表明乙醇氧化反应的活化能最低。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。15 m2/g 水热反应时间 39 h 制备的 Pt/C 合成催化剂,其电催化性能、稳定性和抗中毒性能均最佳。电化学活性表面积为102.83 m2/g,乙醇氧化的峰值电流密度为757.17 A/g,1100 s的稳态电流密度为108.17 A/g,表明乙醇氧化反应的活化能最低。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。17 A/g 显示了乙醇氧化反应的最低活化能。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。17 A/g 显示了乙醇氧化反应的最低活化能。循环伏安图扫描500次循环时,氧化峰电流密度保持率为87.74%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号