当前位置:
X-MOL 学术
›
J. Rare Earths
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical behavior of dysprosium(Ⅲ) in eutectic LiF-DyF3 at tungsten and copper electrodes
Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jre.2019.07.016 Chunfa Liao , Boqing Cai , Xu Wang , Shumei Chen , Gong Chen , Jueyuan Lin
Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jre.2019.07.016 Chunfa Liao , Boqing Cai , Xu Wang , Shumei Chen , Gong Chen , Jueyuan Lin
|
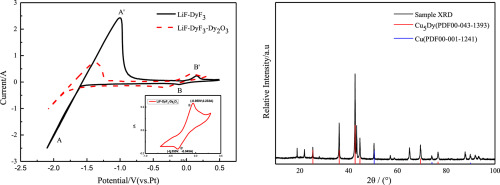
Abstract Electrochemical behavior of dysprosium (Dy) ions in LiF-DyF3 (24 mol%) was investigated by cyclic voltammetry, chronoamperometry and chronopotentiometry. Dy‐Cu alloy samples were prepared by constant-potential electrolysis in LiF-DyF3 (24 mol%) at the Cu electrode. The Cu5Dy and Cu phases were characterized by an X-ray diffractometer and a scanning electron microscope equipped with an energy dispersive spectrometer. The results show that the reduction of Dy(III) ions in a LiF-DyF3 (24 mol%) molten salt system is found to be a quasi-reversible diffusion-controlled process which occurs via a one-step reaction involving the transfer of three electrons. The electro-crystallization processes of the Dy metal at the W electrode and the mode of nucleation confirm that progressive nucleation is dominant at high concentrations of Dy ions in the LiF-DyF3 salt. At lower concentrations, the instantaneous nucleation of Dy with three-dimensional growth of the nuclei is dominant.
中文翻译:

共晶LiF-DyF3中镝(Ⅲ)在钨和铜电极上的电化学行为
摘要 通过循环伏安法、计时电流法和计时电位法研究了 LiF-DyF3 (24 mol%) 中镝 (Dy) 离子的电化学行为。Dy-Cu 合金样品在 LiF-DyF3 (24 mol%) 中通过恒电位电解在 Cu 电极上制备。Cu5Dy 和 Cu 相通过 X 射线衍射仪和配备能量色散光谱仪的扫描电子显微镜进行表征。结果表明,发现 LiF-DyF3 (24 mol%) 熔盐系统中 Dy(III) 离子的还原是一种准可逆扩散控制过程,该过程通过涉及三个转移的一步反应发生。电子。W 电极上 Dy 金属的电结晶过程和成核模式证实,在 LiF-DyF3 盐中高浓度 Dy 离子下,渐进成核占主导地位。在较低浓度下,Dy 的瞬时成核与核的三维生长占主导地位。
更新日期:2020-04-01
中文翻译:

共晶LiF-DyF3中镝(Ⅲ)在钨和铜电极上的电化学行为
摘要 通过循环伏安法、计时电流法和计时电位法研究了 LiF-DyF3 (24 mol%) 中镝 (Dy) 离子的电化学行为。Dy-Cu 合金样品在 LiF-DyF3 (24 mol%) 中通过恒电位电解在 Cu 电极上制备。Cu5Dy 和 Cu 相通过 X 射线衍射仪和配备能量色散光谱仪的扫描电子显微镜进行表征。结果表明,发现 LiF-DyF3 (24 mol%) 熔盐系统中 Dy(III) 离子的还原是一种准可逆扩散控制过程,该过程通过涉及三个转移的一步反应发生。电子。W 电极上 Dy 金属的电结晶过程和成核模式证实,在 LiF-DyF3 盐中高浓度 Dy 离子下,渐进成核占主导地位。在较低浓度下,Dy 的瞬时成核与核的三维生长占主导地位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号