当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extracellular alpha/beta-hydrolase from Paenibacillus species shares structural and functional homology to tobacco salicylic acid binding protein 2.
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.jsb.2020.107496 Rachael C Wilkinson 1 , Rahman Rahman Pour 2 , Shirin Jamshidi 3 , Vilmos Fülöp 1 , Timothy D H Bugg 2
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.jsb.2020.107496 Rachael C Wilkinson 1 , Rahman Rahman Pour 2 , Shirin Jamshidi 3 , Vilmos Fülöp 1 , Timothy D H Bugg 2
Affiliation
|
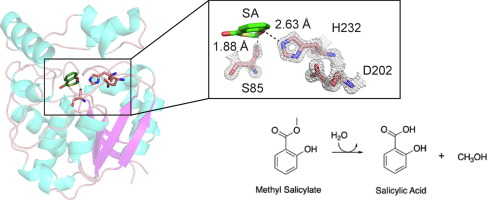
An alpha/ beta hydrolase annotated as a putative salicylate esterase within the genome of a species of Paenibacillus previously identified from differential and selective growth on Kraft lignin was structurally and functionally characterised. Feruloyl esterases are key to the degradation of lignin in several bacterial species and although this activity was investigated, no such activity was observed. The crystal structure of the Paenibacillus esterase, here denoted as PnbE, was determined at 1.32 Å resolution, showing high similarity to Nicotiana tabacum salicylic acid binding protein 2 from the protein database. Structural similarities between these two structures across the core domains and key catalytic residues were observed, with superposition of catalytic residues giving an RMSD of 0.5 Å across equivalent Cα atoms. Conversely, the cap domains of PnbE and Nicotiana tabacum SABP2 showed greater divergence with decreased flexibility in the PnbE cap structure. Activity of PnbE as a putative methyl salicylate esterase was supported with binding studies showing affinity for salicylic acid and functional studies showing methyl salicylate esterase activity. We hypothesise that this activity could enrich Paenibacillus sp. within the rhizosphere by increasing salicylic acid concentrations within the soil.
中文翻译:

芽孢杆菌属物种的细胞外α/β水解酶与烟草水杨酸结合蛋白2具有结构和功能同源性。
在结构和功能上表征了预先从卡夫木质素上的差异和选择性生长鉴定出的一种杆状芽孢杆菌基因组中被标注为推定的水杨酸酯酯酶的α/β水解酶。阿魏酸酯酶是木质素在几种细菌中降解的关键,尽管已研究了这种活性,但未观察到这种活性。以1.32Å的分辨率确定了Paenibacillus酯酶的晶体结构(此处表示为PnbE),显示与蛋白质数据库中的烟草水杨酸结合蛋白2高度相似。观察到这两个结构在核心结构域和关键催化残基之间的结构相似性,催化残基的叠加使等效Cα原子的RMSD为0.5。反过来,PnbE和烟草SABP2的帽结构域显示更大的差异,PnbE帽结构的柔性降低。PnbE作为推定的水杨酸甲酯酯酶的活性得到了对水杨酸亲和力的结合研究和表明水杨酸甲酯酯酶活性的功能研究的支持。我们假设这种活动可以丰富Paenibacillus sp。通过增加土壤中水杨酸的浓度在根际内
更新日期:2020-03-26
中文翻译:

芽孢杆菌属物种的细胞外α/β水解酶与烟草水杨酸结合蛋白2具有结构和功能同源性。
在结构和功能上表征了预先从卡夫木质素上的差异和选择性生长鉴定出的一种杆状芽孢杆菌基因组中被标注为推定的水杨酸酯酯酶的α/β水解酶。阿魏酸酯酶是木质素在几种细菌中降解的关键,尽管已研究了这种活性,但未观察到这种活性。以1.32Å的分辨率确定了Paenibacillus酯酶的晶体结构(此处表示为PnbE),显示与蛋白质数据库中的烟草水杨酸结合蛋白2高度相似。观察到这两个结构在核心结构域和关键催化残基之间的结构相似性,催化残基的叠加使等效Cα原子的RMSD为0.5。反过来,PnbE和烟草SABP2的帽结构域显示更大的差异,PnbE帽结构的柔性降低。PnbE作为推定的水杨酸甲酯酯酶的活性得到了对水杨酸亲和力的结合研究和表明水杨酸甲酯酯酶活性的功能研究的支持。我们假设这种活动可以丰富Paenibacillus sp。通过增加土壤中水杨酸的浓度在根际内



























 京公网安备 11010802027423号
京公网安备 11010802027423号