当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correlations within polyprotein forced unfolding dwell-times introduce sequential dependency.
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.jsb.2020.107495 Einat Chetrit 1 , Yasmine Meroz 2 , Ziv Klausner 3 , Ronen Berkovich 4
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.jsb.2020.107495 Einat Chetrit 1 , Yasmine Meroz 2 , Ziv Klausner 3 , Ronen Berkovich 4
Affiliation
|
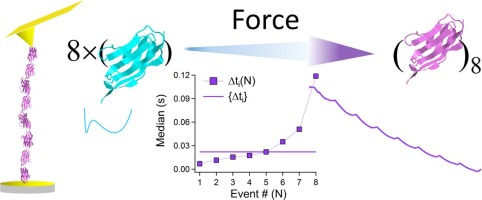
Polyproteins, comprised from proteins arrayed in tandem, respond to mechanical loads through partial unfolding and extension. This response to tension that enables their physiological function is related to the ability to dynamically regulate their elasticity. The unique arrangement of their individual mechanical components (proteins and polymeric linkers), and the interactions between them eventually determines their performance. The sequential unfolding-times within a polyprotein are inherently assumed to be independent and identically distributed (iid), thus expected to follow an exponential distribution. Nevertheless, a large body of literature using single molecule force spectroscopy (SMFS) provides evidence that forced unfolding-times of N proteins within a polyprotein do not follow the exponential distribution. Here we use SMFS with Atomic Force Microscopy to measure the unfolding kinetics of Poly-(I91)8 at 180 pN. The unfolding time-intervals were statistically analysed using three common approaches, all exhibiting an N-effect: hierarchical behavior with non-identical unfolding time distributions. Using continuous time random walk approach indicates that the unfolding times display subdiffusive features. Put together with free-energy reconstruction of the whole unfolding polyprotein, we provide physical explanation for this nontrivial behavior, according to which the elongating polypeptide chain with each unfolding event intervenes with the sequential unfolding probabilities and correlates them.
中文翻译:

多蛋白内强迫展开时间的相关性引入了顺序依赖性。
由串联排列的蛋白质组成的多蛋白通过部分展开和延伸来响应机械负荷。对张力的响应使它们的生理功能得以实现,与动态调节其弹性的能力有关。它们各自的机械成分(蛋白质和聚合物接头)的独特排列方式以及它们之间的相互作用最终决定了它们的性能。固有地假定多蛋白内的顺序展开时间是独立的并且是均匀分布的(iid),因此预期遵循指数分布。然而,使用单分子力谱(SMFS)的大量文献提供了证据,证明多蛋白中N蛋白的强制展开时间不遵循指数分布。在这里,我们将SMFS与原子力显微镜一起使用以测量180 pN处的Poly-(I91)8的展开动力学。使用三种常见的方法对展开时间间隔进行统计分析,所有这些方法均显示出N效应:具有不相同的展开时间分布的分层行为。使用连续时间随机游走方法表明展开时间显示出亚扩散特征。结合整个展开的多蛋白的自由能重建,我们为这种非平凡的行为提供了物理解释,根据该解释,具有每个展开事件的延长多肽链与顺序的展开概率进行干预并将它们相关联。使用三种常见的方法对展开时间间隔进行统计分析,所有这些方法均显示出N效应:具有不相同的展开时间分布的分层行为。使用连续时间随机游走方法表明展开时间显示出亚扩散特征。结合整个展开的多蛋白的自由能重建,我们为这种非平凡的行为提供了物理解释,根据该解释,具有每个展开事件的延长多肽链与顺序的展开概率进行干预并将它们相关联。使用三种常见的方法对展开时间间隔进行统计分析,所有这些方法均显示出N效应:具有不相同的展开时间分布的分层行为。使用连续时间随机游走方法表明展开时间显示出亚扩散特征。结合整个展开的多蛋白的自由能重建,我们为这种非平凡的行为提供了物理解释,根据该解释,具有每个展开事件的延长多肽链与顺序的展开概率进行干预并将它们相关联。
更新日期:2020-03-26
中文翻译:

多蛋白内强迫展开时间的相关性引入了顺序依赖性。
由串联排列的蛋白质组成的多蛋白通过部分展开和延伸来响应机械负荷。对张力的响应使它们的生理功能得以实现,与动态调节其弹性的能力有关。它们各自的机械成分(蛋白质和聚合物接头)的独特排列方式以及它们之间的相互作用最终决定了它们的性能。固有地假定多蛋白内的顺序展开时间是独立的并且是均匀分布的(iid),因此预期遵循指数分布。然而,使用单分子力谱(SMFS)的大量文献提供了证据,证明多蛋白中N蛋白的强制展开时间不遵循指数分布。在这里,我们将SMFS与原子力显微镜一起使用以测量180 pN处的Poly-(I91)8的展开动力学。使用三种常见的方法对展开时间间隔进行统计分析,所有这些方法均显示出N效应:具有不相同的展开时间分布的分层行为。使用连续时间随机游走方法表明展开时间显示出亚扩散特征。结合整个展开的多蛋白的自由能重建,我们为这种非平凡的行为提供了物理解释,根据该解释,具有每个展开事件的延长多肽链与顺序的展开概率进行干预并将它们相关联。使用三种常见的方法对展开时间间隔进行统计分析,所有这些方法均显示出N效应:具有不相同的展开时间分布的分层行为。使用连续时间随机游走方法表明展开时间显示出亚扩散特征。结合整个展开的多蛋白的自由能重建,我们为这种非平凡的行为提供了物理解释,根据该解释,具有每个展开事件的延长多肽链与顺序的展开概率进行干预并将它们相关联。使用三种常见的方法对展开时间间隔进行统计分析,所有这些方法均显示出N效应:具有不相同的展开时间分布的分层行为。使用连续时间随机游走方法表明展开时间显示出亚扩散特征。结合整个展开的多蛋白的自由能重建,我们为这种非平凡的行为提供了物理解释,根据该解释,具有每个展开事件的延长多肽链与顺序的展开概率进行干预并将它们相关联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号