Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-02-22 , DOI: 10.1016/j.csbj.2020.02.005 Abhijit Dasgupta 1 , Gautam K Bandyopadhyay 2 , Indrani Ray 3 , Keya Bandyopadhyay 2 , Nirmalya Chowdhury 4 , Rajat K De 3 , Sushil K Mahata 2, 5
|
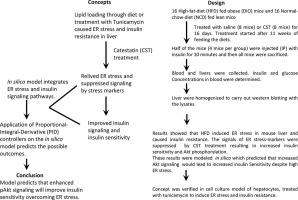
Obesity is characterized by a state of chronic, unresolved inflammation in insulin-targeted tissues. Obesity-induced inflammation causes accumulation of proinflammatory macrophages in adipose tissue and liver. Proinflammatory cytokines released from tissue macrophages inhibits insulin sensitivity. Obesity also leads to inflammation-induced endoplasmic reticulum (ER) stress and insulin resistance. In this scenario, based on the data (specifically patterns) generated by our in vivo experiments on both diet-induced obese (DIO) and normal chow diet (NCD) mice, we developed an in silico state space model to integrate ER stress and insulin signaling pathways. Computational results successfully followed the experimental results for both DIO and NCD conditions. Chromogranin A (CgA) peptide catestatin (CST: ) improves obesity-induced hepatic insulin resistance by reducing inflammation and inhibiting proinflammatory macrophage infiltration. We reasoned that the anti-inflammatory effects of CST would alleviate ER stress. CST decreased obesity-induced ER dilation in hepatocytes and macrophages. On application of Proportional-Integral-Derivative (PID) controllers on the in silico model, we checked whether the reduction of phosphorylated PERK resulting in attenuation of ER stress, resembling CST effect, could enhance insulin sensitivity. The simulation results clearly pointed out that CST not only decreased ER stress but also enhanced insulin sensitivity in mammalian cells. In vivo experiment validated the simulation results by depicting that CST caused decrease in phosphorylation of UPR signaling molecules and increased phosphorylation of insulin signaling molecules. Besides simulation results predicted that enhancement of AKT phosphorylation helps in both overcoming ER stress and achieving insulin sensitivity. These effects of CST were verified in hepatocyte culture model.
中文翻译:

Catestatin可通过减轻内质网应激来提高胰岛素敏感性:体内和计算机验证。
肥胖症的特征在于胰岛素靶向组织中的慢性,未解决的炎症状态。肥胖引起的炎症会导致促炎性巨噬细胞在脂肪组织和肝脏中积累。从组织巨噬细胞释放的促炎细胞因子抑制胰岛素敏感性。肥胖还导致炎症引起的内质网(ER)应激和胰岛素抵抗。在这种情况下,根据我们对饮食诱发的肥胖(DIO)和正常食物(NCD)小鼠进行的体内实验所产生的数据(特定模式),我们开发了计算机模拟状态空间模型来整合内质网应激和胰岛素信号通路。在DIO和NCD条件下,计算结果均成功地跟随了实验结果。嗜铬粒蛋白A(CgA)肽catestatin(CST:)通过减少炎症和抑制炎症性巨噬细胞浸润来改善肥胖引起的肝胰岛素抵抗。我们认为,CST的抗炎作用将缓解内质网应激。CST减少了肥胖引起的肝细胞和巨噬细胞内质网扩张。在比例积分微分(PID)控制器在计算机模型上的应用中,我们检查了磷酸化PERK的减少是否导致ER应力减弱(类似于CST效应)是否可以增强胰岛素敏感性。模拟结果清楚地表明,CST不仅降低了ER应激,而且增强了哺乳动物细胞中的胰岛素敏感性。体内实验通过描述CST引起UPR信号分子的磷酸化减少和胰岛素信号分子的磷酸化增加,验证了仿真结果。此外,模拟结果还预测,增强AKT磷酸化有助于克服ER应激和实现胰岛素敏感性。在肝细胞培养模型中证实了CST的这些作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号