Materials Today Energy ( IF 9.0 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.mtener.2020.100387 Ziqi Zhang , Linchuan Cong , Zhuochen Yu , Lina Qu , Weimin Huang
|
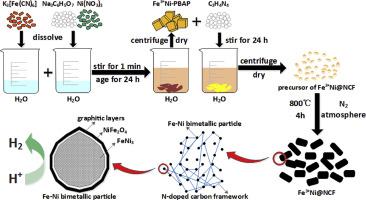
Hydrogen is one of the most desirable sources with large energy density. Electrocatalysis of hydrogen evolution reaction is a promising way to produce hydrogen since it can be powered by renewable energy such as solar and wind energy. However, the mainstream electrocatalyst is noble metal (Pt/Ru/Ir), which is expensive and rare. It is necessary to develop non-noble metal catalysts toward HER from the perspective of sustainable development and long-term environmental conservation. Therefore, this paper provides a cheap and feasible way to synthesize the Fe–Ni bimetallic N-doped carbon framework (Fe–Ni@NCF). The prepared Fe–Ni@NCF exhibits the desired overpotential of 219 mV at 10 mA cm−2 in alkaline and 259 mV at 10 mA cm−2 in acidic solution, respectively. Besides, it exhibits similar current density loss as commercial Pt/C after 10,000 segments of CVs, which suggests satisfactory durability.
中文翻译:

Fe-Ni双金属氮掺杂碳骨架的简便合成,可实现高效的电化学制氢反应
氢是具有高能量密度的最理想的来源之一。氢气析出反应的电催化是一种有前途的生产氢气的方法,因为它可以由太阳能和风能等可再生能源提供动力。但是,主流的电催化剂是贵金属(Pt / Ru / Ir),价格昂贵且稀有。从可持续发展和长期环境保护的角度出发,有必要开发针对HER的非贵金属催化剂。因此,本文为合成Fe-Ni双金属N掺杂碳骨架(Fe-Ni @ NCF)提供了一种廉价而可行的方法。将制备的Fe-Ni @ NCF表现出在10mA厘米所需的超电势219毫伏-2在碱性和259毫伏在10mA厘米-2分别在酸性溶液中。此外,在10,000个CV分段后,它的电流密度损失与商用Pt / C相似,这表明令人满意的耐久性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号