Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.xcrp.2020.100033 Mohammad Rahimi , Giulia Catalini , Subrahmaniam Hariharan , Miao Wang , Monica Puccini , T. Alan Hatton
|
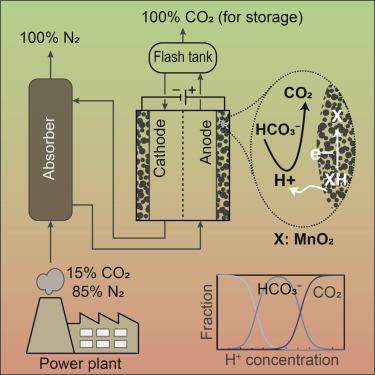
The development of sustainable CO2 capture technologies is critical to address issues associated with global warming. In this context, the concept of an electrochemically driven proton concentration process is developed for the capture of CO2 based on modulation of the proton concentration in an electrochemical cell by a proton intercalating MnO2 electrode. The pH sensitivity of CO2 hydration is leveraged such that CO2 is absorbed as bicarbonate and carbonate ions at high pH values and desorbed as gas at low pH values. The electrochemical work requirement for the proposed proton concentration process to desorb CO2 captured from a flue gas stream is estimated to be 33.2 kJe/mol CO2, suggesting that this process is competitive with other similar electrochemical-based approaches. The experimental results show that the generated current in a symmetrical electrochemical cell with fabricated electrodes is effectively translated into proton intercalation/deintercalation reactions through reversible cycles, resulting in modulated proton concentrations.
中文翻译:

使用电化学驱动质子浓缩过程捕获二氧化碳
可持续的CO 2捕集技术的发展对于解决与全球变暖有关的问题至关重要。在此背景下,基于质子嵌入MnO 2电极对电化学池中质子浓度的调节,开发了一种电化学驱动质子浓缩过程的概念,用于捕获CO 2。利用CO 2水合的pH敏感性,使得CO 2在高pH值下作为碳酸氢根和碳酸根离子被吸收,而在低pH值下作为气体被解吸。拟议的质子浓缩过程解吸从烟气流中捕获的CO 2的电化学工作要求估计为33.2 kJe / mol CO如图2所示,表明该方法与其他类似的基于电化学的方法竞争。实验结果表明,在具有装配好的电极的对称电化学电池中,通过可逆循环可有效地将其产生的电流转化为质子嵌入/脱嵌反应,从而调节质子浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号