当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expedient Synthesis of Lupulones and Their Derivatization to 2,8‐7H‐Dihydrochromen‐7‐ones
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-04-06 , DOI: 10.1002/open.202000008 Lena Decuyper 1 , Gurkirat Kaur 1 , Charlotte Versyck 1 , Eline Blondeel 1 , Yves Depetter 1 , Kristof Van Hecke 2 , Matthias D'hooghe 1
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-04-06 , DOI: 10.1002/open.202000008 Lena Decuyper 1 , Gurkirat Kaur 1 , Charlotte Versyck 1 , Eline Blondeel 1 , Yves Depetter 1 , Kristof Van Hecke 2 , Matthias D'hooghe 1
Affiliation
|
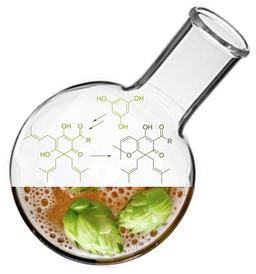
A convenient and improved method for the synthesis of beta acids or lupulones, which are known to possess e. g. anti‐cancer, anti‐inflammatory, anti‐oxidative and antimicrobial activity, has been developed successfully. Further derivatization of these complex structures to the corresponding dihydrochromen‐7‐ones, including the natural product machuone, was realized to simplify their analysis and to confirm their molecular structure. In addition to practical and safe laboratory procedures, the advantages associated with this new approach involve the use of water as a solvent and the direct crystallization of lupunones from acetonitrile, rendering our strategy more efficient and benign as compared to available methods.
中文翻译:

蛇麻酮的便捷合成及其衍生化为 2,8-7H-二氢色烯-7-酮
一种合成 β 酸或蛇麻酮的方便且改进的方法,已知这些酸或蛇麻酮具有e.克。抗癌、抗炎、抗氧化和抗菌活性已被开发成功。这些复杂结构进一步衍生为相应的二氢色烯-7-酮,包括天然产物马丘酮,以简化其分析并确认其分子结构。除了实用和安全的实验室程序之外,与这种新方法相关的优点包括使用水作为溶剂以及从乙腈中直接结晶羽扇豆酮,使我们的策略与现有方法相比更加有效和良性。
更新日期:2020-04-06
中文翻译:

蛇麻酮的便捷合成及其衍生化为 2,8-7H-二氢色烯-7-酮
一种合成 β 酸或蛇麻酮的方便且改进的方法,已知这些酸或蛇麻酮具有e.克。抗癌、抗炎、抗氧化和抗菌活性已被开发成功。这些复杂结构进一步衍生为相应的二氢色烯-7-酮,包括天然产物马丘酮,以简化其分析并确认其分子结构。除了实用和安全的实验室程序之外,与这种新方法相关的优点包括使用水作为溶剂以及从乙腈中直接结晶羽扇豆酮,使我们的策略与现有方法相比更加有效和良性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号