当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of diazine-based panobinostat analogues for HDAC8 inhibition.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-04-07 , DOI: 10.3762/bjoc.16.59 Sivaraman Balasubramaniam 1 , Sajith Vijayan 1 , Liam V Goldman 2 , Xavier A May 2 , Kyra Dodson 1 , Sweta Adhikari 1 , Fatima Rivas 3 , Davita L Watkins 1 , Shana V Stoddard 2
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-04-07 , DOI: 10.3762/bjoc.16.59 Sivaraman Balasubramaniam 1 , Sajith Vijayan 1 , Liam V Goldman 2 , Xavier A May 2 , Kyra Dodson 1 , Sweta Adhikari 1 , Fatima Rivas 3 , Davita L Watkins 1 , Shana V Stoddard 2
Affiliation
|
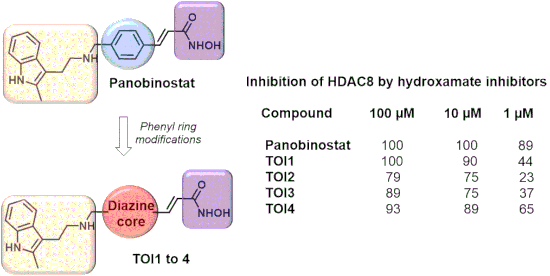
Guided by computational analysis, herein we report the design, synthesis and evaluation of four novel diazine-based histone deacetylase inhibitors (HDACis). The targets of interest (TOI) are analogues of panobinostat, one of the most potent and versatile HDACi reported. By simply replacing the phenyl core of panobinostat with that of a diazine derivative, docking studies against HDAC2 and HDAC8 revealed that the four analogues exhibit inhibition activities comparable to that of panobinostat. Multistep syntheses afforded the visualized targets TOI1, TOI2, TOI3-rev and TOI4 whose biological evaluation confirmed the strength of HDAC8 inhibition with TOI4 displaying the greatest efficacy at varying concentrations. The results of this study lay the foundation for future design strategies toward more potent HDACis for HDAC8 isozymes and further therapeutic applications for neuroblastoma.
中文翻译:

设计和合成用于HDAC8抑制的基于二嗪的panobinostat类似物。
在计算分析的指导下,本文报道了四种新型的基于二嗪的组蛋白脱乙酰基酶抑制剂(HDACis)的设计,合成和评估。感兴趣的靶标(TOI)是panobinostat的类似物,panobinostat是报道的最有效且用途最广泛的HDACi之一。通过简单地用二嗪衍生物替换panobinostat的苯基核心,对HDAC2和HDAC8的对接研究表明,四种类似物的抑制活性与panobinostat相当。多步合成提供了可视化的目标TOI1,TOI2,TOI3-rev和TOI4,其生物学评估证实了HDAC8抑制的强度,TOI4在不同浓度下显示出最大的功效。
更新日期:2020-04-08
中文翻译:

设计和合成用于HDAC8抑制的基于二嗪的panobinostat类似物。
在计算分析的指导下,本文报道了四种新型的基于二嗪的组蛋白脱乙酰基酶抑制剂(HDACis)的设计,合成和评估。感兴趣的靶标(TOI)是panobinostat的类似物,panobinostat是报道的最有效且用途最广泛的HDACi之一。通过简单地用二嗪衍生物替换panobinostat的苯基核心,对HDAC2和HDAC8的对接研究表明,四种类似物的抑制活性与panobinostat相当。多步合成提供了可视化的目标TOI1,TOI2,TOI3-rev和TOI4,其生物学评估证实了HDAC8抑制的强度,TOI4在不同浓度下显示出最大的功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号