Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-04-06 , DOI: 10.1016/j.cej.2020.124912 Jiaojie He , Yuhong Xu , Penghui Shao , Liwei Yang , Yan Sun , Yue Yang , Fuyi Cui , Wei Wang
|
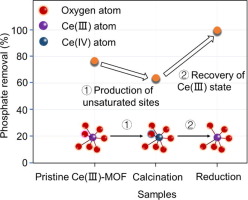
The electronic structure and associated chemical characteristics of metal-based adsorbent are directly relevant to the selectivity and efficiency of phosphate uptake. However, few studies focus on the nature of metal centers’ electronic orbit (i.e., the coordination number and valence state). Herein, we report a coordinatively unsaturated Ce(III)-based materials, which exhibits excellent potential in effective phosphate removal. Via controlled partial thermolysis and the following reduction process, the valence state and coordination number of original Ce(III)-MOF (denoted as CM) can be tuned and optimized. The manufacture of more coordination vacancy was fulfilled through total release of solvent molecules under annealing at 300 oC in air. Meanwhile, the reduction procedure could precisely tuned the ratio of Ce(III)/Ce(IV). The result shows that samples only annealed induce a sharp decrease of the phosphate capacity due to the high amount of Ce(IV) state. After the reduction process, the XPS spectra reveal the growth of oxygen vacancy content calculated as 11.6 % and the increase of Ce(III)/Ce(IV) values from 0.79:1 to 1.36:1. Based on those great improvement of the unsaturated coordination numbers and the recovery of Ce(III) content for metal centers, the maximum capacity of CM-300(R) to adsorb phosphate is up to 273 mg/g, 2.6 times larger than that of pristine CM. Those new insights provide a novel strategy for synthesizing a highly active adsorbent by controlling the electronic structure of metal centers for efficient phosphate removal.
中文翻译:

铈基吸附剂的配位不饱和度和价态的调节以促进磷酸盐的吸附
金属基吸附剂的电子结构和相关的化学特性与磷酸盐吸收的选择性和效率直接相关。但是,很少有研究关注金属中心电子轨道的性质(即配位数和价态)。在本文中,我们报道了一种配位不饱和的Ce(III)基材料,在有效去除磷酸盐方面显示出极好的潜力。通过控制部分热解和随后的还原过程,可以调节和优化原始Ce(III)-MOF(表示为CM)的化合价态和配位数。通过在300 o退火下完全释放溶剂分子来完成更多配位空位的制造C在空气中。同时,还原过程可以精确地调节Ce(III)/ Ce(IV)的比例。结果表明,由于大量的Ce(IV)状态,仅退火的样品会引起磷酸盐容量的急剧下降。还原过程后,XPS光谱显示,氧空位含量的增长为11.6%,Ce(III)/ Ce(IV)值从0.79:1增加到1.36:1。基于金属中心的不饱和配位数的大幅提高和Ce(III)含量的回收,CM-300(R)的最大吸附磷量高达273 mg / g,是金属的2.6倍。原始CM。这些新见解通过控制金属中心的电子结构以有效去除磷酸盐,为合成高活性吸附剂提供了一种新颖的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号