Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-04-06 , DOI: 10.1016/j.cej.2020.124914 Ana Bjelić , Miha Grilc , Blaž Likozar
|
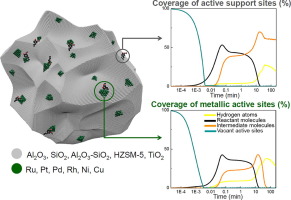
The hydrodeoxygenation (HDO) reaction kinetics of lignin monomer model compound eugenol was systematically investigated over various commercially available catalysts typically used for lignin valorisation by hydrotreatment. The role of noble metals Pt, Pd, Rh, Ru, and non-noble Ni and Cu on C was investigated in the previous studies, while the present work is focused on the support (Al2O3, SiO2, SiO2-Al2O3, TiO2, HZSM-5) effect on the catalyst activity and selectivity, being estimated experimentally (at 225, 275, 325 °C and 5 MPa of initial hydrogen pressure) and in silico. The micro-kineticmodel took into consideration mass transfer resistances through convective films (gas bubbles and catalyst particles), reaction kinetics in bulk liquid, adsorption/desorption and surface reaction kinetics on metallic and acidic active sites, considering the occurrence of all reactions on both types of sites. The contribution of each support was quantitatively evaluated in terms of adsorption, desorption, and reaction rate constants and activation energies. SiO2-Al2O3 and HZSM-5 showed superior contribution to the activity of the methoxyl and hydroxyl group removal from oxygenated aromatics and particularly saturated species. Except for the above-mentioned materials Caromatic–O hydrogenolysis activity was not substantially affected, while the acidity of the support notably promoted Caliphatic–O cleavage. Contribution to overall hydrogenation rate was practically negligible in presence of acidic sites. It has been also noted that the contribution of supports was not very prominent when very active noble metal phases were used (such as Ru), while it came to the fore in the case of almost inactive Cu, being responsible for formation of 4-propylphenol at 325 °C. Based on microkinetic modelling results, Sankey diagrams were formulated for the first time to represent pathway flux and graphically demonstrate the contribution of each catalytic reaction participating in the overall eugenol HDO mechanism over the used catalysts (and corresponding intermediates) within a complex reaction network, as well as the engagement of metallic and acidic sites.
中文翻译:

Pt,Pd,Rh,Ru,Ni和Cu催化剂上丁香酚作为木质素模型化合物加氢脱氧的双功能金属-酸性机理
木质素单体模型化合物丁子香酚的加氢脱氧(HDO)反应动力学是通过通常用于加氢处理木质素增值的各种市售催化剂系统研究的。基于C的贵金属铂,钯,铑,钌,和非贵金属Ni和Cu的作用,在以前的研究中进行了研究,而目前的工作集中在支撑体(铝2 ö 3的SiO 2的SiO 2 -的Al 2 Ó 3,二氧化钛2,HZSM-5)的催化剂活性和选择性的影响,被实验估计(在225,275,325℃和初始氢气压力为5兆帕)和在计算机芯片上。微观动力学模型考虑了通过对流膜(气泡和催化剂颗粒)的传质阻力,本体液体中的反应动力学,金属和酸性活性位上的吸附/解吸和表面反应动力学,同时考虑了两种类型的所有反应网站。根据吸附,解吸,反应速率常数和活化能对每种载体的贡献进行定量评估。SiO 2 -Al 2 O 3和HZSM-5对从含氧芳族化合物特别是饱和物质中除去甲氧基和羟基的活性表现出优异的贡献。除上述材料C以外的芳香族-O的氢解活性基本上没有受到影响,而载体的酸度显着提高了C脂肪族–O分裂。在酸性位点的存在下,对总氢化率的贡献实际上可以忽略不计。还已经注意到,当使用非常活泼的贵金属相(例如Ru)时,载体的贡献不是很明显,而对于几乎不活泼的Cu来说,它的作用才是重要的,它负责形成4-丙基苯酚在325°C下。根据微观动力学建模结果,首次编制了Sankey图以表示途径通量,并以图形方式显示了参与整个丁香酚HDO机理的每个催化反应对复杂反应网络中所用催化剂(和相应中间体)的贡献,以及金属和酸性部位的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号